Targeting thapsigargin towards tumors
- PMID: 25065587
- PMCID: PMC4696022
- DOI: 10.1016/j.steroids.2014.07.009
Targeting thapsigargin towards tumors
Abstract
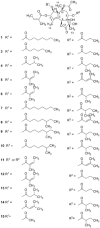
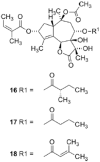
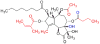
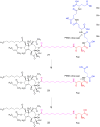
The skin irritating principle from Thapsia garganica was isolated, named thapsigargin and the structure elucidated. By inhibiting the sarco/endoplasmic reticulum Ca(2+) ATPase (SERCA) thapsigargin provokes apoptosis in almost all cells. By conjugating thapsigargin to peptides, which are only substrates for either prostate specific antigen (PSA) or prostate specific membrane antigen (PSMA) prodrugs were created, which selectively affect prostate cancer cells or neovascular tissue in tumors. One of the prodrug is currently tested in clinical phase II. The prodrug under clinical trial has been named mipsagargin.
Keywords: Prodrug; Prostate specific antigen (PSA); Prostate specific membrane antigen (PSMA); Sarco/endoplasmic reticulum (SERCA); Targeting drugs; Thapsigargin.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





References
-
- Perrot E. Matière Première usuelle du Règne Vègètale. Paris: Masson et cie; 1943. pp. 1630–2.
-
- Rasmussen U, Christensen SB, Sandberg F. Thapsigargin and Thapsigargicin, two new histamine liberators from Thapsia garganica L. Acta Pharm Suec. 1978;15:133–40. - PubMed
-
- Christensen SB, Rasmussen U, Christophersen C. Thapsigargin, constitution of a sesquiterpene lactone histamine liberator from Thapsia garganica. Tetrahedron Lett. 1980;21:3829–30.
-
- Christensen SB, Larsen IK, Rasmussen U, Christophersen C. Thapsigargin and thapsigargicin, two histamine liberating sesquiterpene lactones from Thapsia garganica. X-ray analysis of the 7,11-epoxide of thapsigargin. J Org Chem. 1982;47:649–52.
-
- Holub M, Samek Z, deGroote R, Herout V, Sorm F. On terpenes. CCXXVII. The structure of the sesquiterpene triester lactone trilobolide. Collect Czech Chem Commun. 1973;38:1551–62.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

