Orthogonal functionalisation of α-helix mimetics
- PMID: 25065821
- PMCID: PMC4157654
- DOI: 10.1039/c4ob00915k
Orthogonal functionalisation of α-helix mimetics
Abstract
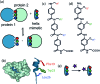
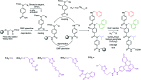
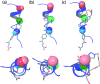
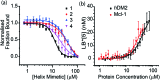
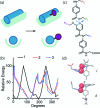
α-Helix mediated protein-protein interactions are of major therapeutic importance. As such, the design of inhibitors of this class of interaction is of significant interest. We present methodology to modify N-alkylated aromatic oligoamide α-helix mimetics using 'click' chemistry. The effect is shown to modulate the binding properties of a series of selective p53/hDM2 inhibitors.
Figures

References
-
- Milroy L.-G., Grossmann T. N., Hennig S., Brunsveld L., Ottmann C. Chem. Rev. 2014;114:4695–4748. - PubMed
-
- Aeluri M., Chamakuri S., Dasari B., Guduru S. K. R., Jimmidi R., Jogula S., Arya P. Chem. Rev. 2014;114:4640–4694. - PubMed
-
- Wells J. A., McClendon C. L. Nature. 2007;450:1001–1009. - PubMed
-
- Keskin O., Gursoy A., Ma B., Nussinov R. Chem. Rev. 2008;108:1225–1244. - PubMed
-
- Clackson T., Wells J. A. Science. 1995;267:383–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

