Selective responses to tonic descending commands by temporal summation in a spinal motor pool
- PMID: 25066087
- PMCID: PMC4126198
- DOI: 10.1016/j.neuron.2014.06.021
Selective responses to tonic descending commands by temporal summation in a spinal motor pool
Abstract
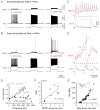
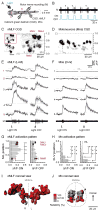
Motor responses of varying intensities rely on descending commands to heterogeneous pools of motoneurons. In vertebrates, numerous sources of descending excitatory input provide systematically more drive to progressively less excitable spinal motoneurons. While this presumably facilitates simultaneous activation of motor pools, it is unclear how selective patterns of recruitment could emerge from inputs weighted this way. Here, using in vivo electrophysiological and imaging approaches in larval zebrafish, we find that, despite weighted excitation, more excitable motoneurons are preferentially activated by a midbrain reticulospinal nucleus by virtue of longer membrane time constants that facilitate temporal summation of tonic drive. We confirm the utility of this phenomenon by assessing the activity of the midbrain and motoneuron populations during a light-driven behavior. Our findings demonstrate that weighted descending commands can generate selective motor responses by exploiting systematic differences in the biophysical properties of target motoneurons and their relative sensitivity to tonic input.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures






References
-
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–578. - PubMed
-
- Bhatt DH, McLean DL, Hale ME, Fetcho JR. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. - PubMed
-
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

