Heterogeneous tumor subpopulations cooperate to drive invasion
- PMID: 25066122
- PMCID: PMC4542310
- DOI: 10.1016/j.celrep.2014.06.045
Heterogeneous tumor subpopulations cooperate to drive invasion
Abstract
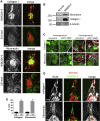
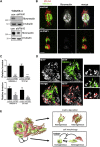
Clonal selection and transcriptional reprogramming (e.g., epithelial-mesenchymal transition or phenotype switching) are the predominant theories thought to underlie tumor progression. However, a "division of labor" leading to cooperation among tumor-cell subpopulations could be an additional catalyst of progression. Using a zebrafish-melanoma xenograft model, we found that in a heterogeneous setting, inherently invasive cells, which possess protease activity and deposit extracellular matrix (ECM), co-invade with subpopulations of poorly invasive cells, a phenomenon we term "cooperative invasion". Whereas the poorly invasive cells benefit from heterogeneity, the invasive cells switch from protease-independent to an MT1-MMP-dependent mode of invasion. We did not observe changes in expression of the melanoma phenotype determinant MITF during cooperative invasion, thus ruling out the necessity for phenotype switching for invasion. Altogether, our data suggest that cooperation can drive melanoma progression without the need for clonal selection or phenotype switching and can account for the preservation of heterogeneity seen throughout tumor progression.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
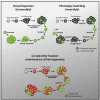
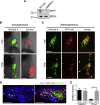
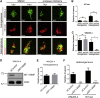
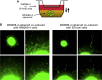
Comment in
-
Going forward together: cooperative invasion in melanoma.Pigment Cell Melanoma Res. 2015 Jan;28(1):6-7. doi: 10.1111/pcmr.12323. Epub 2014 Nov 10. Pigment Cell Melanoma Res. 2015. PMID: 25308321 No abstract available.
References
-
- Friedland J.C., Lee M.H., Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. - PubMed
-
- Gaggioli C., Deckert M., Robert G., Abbe P., Batoz M., Ehrengruber M.U., Ortonne J.P., Ballotti R., Tartare-Deckert S. HGF induces fibronectin matrix synthesis in melanoma cells through MAP kinase-dependent signaling pathway and induction of Egr-1. Oncogene. 2005;24:1423–1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

