Biomaterials in myocardial tissue engineering
- PMID: 25066525
- PMCID: PMC4933503
- DOI: 10.1002/term.1944
Biomaterials in myocardial tissue engineering
Abstract
Cardiovascular disease is the leading cause of death in the developed world, and as such there is a pressing need for treatment options. Cardiac tissue engineering emerged from the need to develop alternative sources and methods of replacing tissue damaged by cardiovascular diseases, as the ultimate treatment option for many who suffer from end-stage heart failure is a heart transplant. In this review we focus on biomaterial approaches to augmenting injured or impaired myocardium, with specific emphasis on: the design criteria for these biomaterials; the types of scaffolds - composed of natural or synthetic biomaterials or decellularized extracellular matrix - that have been used to develop cardiac patches and tissue models; methods to vascularize scaffolds and engineered tissue; and finally, injectable biomaterials (hydrogels) designed for endogenous repair, exogenous repair or as bulking agents to maintain ventricular geometry post-infarct. The challenges facing the field and obstacles that must be overcome to develop truly clinically viable cardiac therapies are also discussed.
Keywords: biomaterials; cardiac regeneration; cardiac scaffolds; cardiac tissue engineering; cardiac tissue models; injectable hydrogels.
Copyright © 2014 John Wiley & Sons, Ltd.
Figures

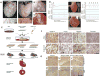

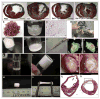
References
-
- Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Pt C-Meth. 2011;17:915–926. - PubMed
-
- Amir G, Miller L, Shachar M, Feinberg MS, Holbova R, Cohen S, Leor J. Evaluation of a peritoneal-generated cardiac patch in a rat model of heterotopic heart transplantation. Cell Transplant. 2009;18:275–282. - PubMed
-
- Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. - PubMed
-
- Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP. Substrates for cardiovascular tissue engineering. Advanced drug delivery reviews. 2011;63:221–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

