Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations
- PMID: 25066951
- PMCID: PMC4124772
- DOI: 10.1186/1471-2431-14-193
Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations
Abstract
Background: Vancomycin is effective against gram-positive bacteria and the first-line antibiotic for treatment of proven coagulase-negative staphylococcal infections. The aim of this study is bipartite: first, to assess the percentage of therapeutic initial trough serum concentrations and second, to evaluate the adequacy of the therapeutic range in interrelationship with the observed MIC-values in neonates.
Methods: In this study, preterm and term neonates admitted at a tertiary NICU in the Netherlands from January 2009 to December 2012 and treated with vancomycin for a proven gram-positive infection were included. Trough serum concentrations were measured prior to administration of the 5th dose. Trough concentrations in the range of 10 to 15 mg/L were considered therapeutic. Staphylococcal species minimal inhibitory concentrations (MIC's) were determined using the E-test method. Species identification was performed by matrix-assisted laser desorption/ionisation mass spectrometry.
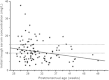
Results: Of the 112 neonates, 53 neonates (47%) had sub-therapeutic initial trough serum concentrations of vancomycin, whereas 22% had supra-therapeutic initial trough serum concentrations. In all patients doses were adjusted on basis of the initial trough concentration. In 40% (23/57) of the neonates the second trough concentration remained sub-therapeutic. MIC's were determined for 30 coagulase-negative Staphylococcus isolates obtained from 19 patients. Only 4 out of 19 subjects had a trough concentration greater than tenfold the MIC.
Conclusions: Forty-seven percent of the neonates had sub-therapeutic initial trough serum concentrations of vancomycin. The MIC-data indicate that the percentages of underdosed patients may be greater. It may be advisable to increase the lower limit of the therapeutic range for European neonates.
Figures
References
-
- Fanos V, Dall’Agnola A. Antibiotics in neonatal infections: a review. Drugs. 1999;58(Suppl 3):405–427. - PubMed
-
- Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988–1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000;106(Suppl 6):1387–1390. - PubMed
-
- Yeung CY, Lee HC, Huang FY, Wang CS. Sepsis during total parenteral nutrition: exploration of risk factors and determination of the effectiveness of peripherally inserted central venous catheters. Pediatr Infect Dis J. 1998;17(Suppl 2):135–142. - PubMed
-
- Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med. 2013;18(Suppl 1):28–34. - PubMed
-
- Rubin LG, Sanchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR. Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists’ practices. Pediatrics. 2002;110(Suppl 4):e42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

