Prevalence of lipodystrophy and metabolic abnormalities in HIV-infected African children after 3 years on first-line antiretroviral therapy
- PMID: 25068287
- PMCID: PMC4369579
- DOI: 10.1097/INF.0000000000000491
Prevalence of lipodystrophy and metabolic abnormalities in HIV-infected African children after 3 years on first-line antiretroviral therapy
Abstract
Background: Most pediatric lipodystrophy data come from high-income/middle-income countries, but most HIV-infected children live in sub-Saharan Africa, where lipodystrophy studies have predominantly investigated stavudine-based regimens.
Methods: Three years after antiretroviral therapy (ART) initiation, body circumferences and skinfold thicknesses were measured (n = 590), and fasted lipid profile assayed (n = 325), in children from 2 ARROW trial centres in Uganda/Zimbabwe. Analyses compared randomization to long-term versus short-term versus no zidovudine from ART initiation [unadjusted; latter 2 groups receiving abacavir+lamivudine+non-nucleoside-reverse-transciptase-inhibitor (nNRTI) long-term], and nonrandomized (confounder-adjusted) receipt of nevirapine versus efavirenz.
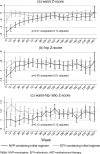
Results: Body circumferences and skinfold thicknesses were similar regardless of zidovudine exposure (P > 0.1), except for subscapular and supra-iliac skinfolds-for-age which were greater with long-term zidovudine (0.006 < P < 0.047). Circumferences/skinfolds were also similar with efavirenz and nevirapine (adjusted P > 0.09; 0.02 < P < 0.03 for waist/waist-hip-ratio). Total and high-density lipoprotein (HDL)-cholesterol, HDL/triglyceride-ratio (P < 0.0001) and triglycerides (P = 0.01) were lower with long-term zidovudine. Low-density lipoprotein (LDL)-cholesterol was higher with efavirenz than nevirapine (P < 0.001). Most lipids remained within normal ranges (75% cholesterol, 85% LDL and 100% triglycerides) but more on long-term zidovudine (3 NRTI) had abnormal HDL-cholesterol (88% vs. 40% short/no-zidovudine, P < 0.0001). Only 8/579(1.4%) children had clinical fat wasting (5 grade 1; 3 grade 2); 2(0.3%) had grade 1 fat accumulation.
Conclusions: Long-term zidovudine-based ART is associated with similar body circumferences and skinfold thicknesses to abacavir-based ART, with low rates of lipid abnormalities and clinical lipodystrophy, providing reassurance where national programs now recommend long-term zidovudine. Efavirenz and nevirapine were also similar; however, the higher LDL observed with efavirenz and lower HDL observed with zidovudine suggests that zidovudine+lamivudine+efavirenz should be investigated in future.
Figures
References
-
- Amaya RA, Kozinetz CA, McMeans A, et al. Lipodystrophy syndrome in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21:405–410. - PubMed
-
- Aurpibul L, Puthanakit T, Lee B, et al. Lipodystrophy and metabolic changes in HIV-infected children on non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Antivir Ther. 2007;12:1247–1254. - PubMed
-
- Badillo K, Prieto L, Toledano M, et al. Characteristics of human immunodeficiency virus-1 infected children receiving highly active antiretroviral therapy: a cross-sectional study. An Pediatr (Barc) 2012;76:317–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical