Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated troglitazone hepatotoxicity
- PMID: 25068506
- PMCID: PMC4480860
- DOI: 10.1038/clpt.2014.158
Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated troglitazone hepatotoxicity
Abstract
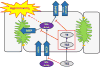
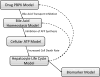
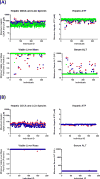
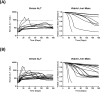
Troglitazone (TGZ) causes delayed, life-threatening drug-induced liver injury in some patients but was not hepatotoxic in rats. This study investigated altered bile acid homeostasis as a mechanism of TGZ hepatotoxicity using a systems pharmacology model incorporating drug/metabolite disposition, bile acid physiology/pathophysiology, hepatocyte life cycle, and liver injury biomarkers. In the simulated human population, TGZ (200-600 mg/day × 6 months) resulted in delayed increases in serum alanine transaminase >3× the upper limit of normal in 0.3-5.1%, with concomitant bilirubin elevations >2× the upper limit of normal in 0.3-3.6%, of the population. By contrast, pioglitazone (15-45 mg/day × 6 months) did not elicit hepatotoxicity, consistent with clinical data. TGZ was not hepatotoxic in the simulated rat population. In summary, mechanistic modeling based only on bile acid effects accurately predicted the incidence, delayed presentation, and species differences in TGZ hepatotoxicity, in addition to predicting the relative liver safety of pioglitazone. Systems pharmacology models integrating physiology and experimental data can evaluate drug-induced liver injury mechanisms and may be useful to predict the hepatotoxic potential of drug candidates.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Comment in
-
Application of systems pharmacology to explore mechanisms of hepatotoxicity.Clin Pharmacol Ther. 2014 Nov;96(5):536-7. doi: 10.1038/clpt.2014.167. Clin Pharmacol Ther. 2014. PMID: 25336266
References
-
- Temple RJ, Himmel MH. Safety of newly approved drugs: implications for prescribing. JAMA. 2002;287:2273–5. - PubMed
-
- Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916–7. - PubMed
-
- Presented at the Endocrinologic and Metabolic Drugs Advisory Committee Meeting; 1999. Aprial 26. Available from the US Food and Drug Administration ( http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3499t1a.pdf). Accessed January 25, 2014.
-
- Isley WL. Hepatotoxicity of thiazolidinediones. Expert Opin Drug Saf. 2003;2:581–6. - PubMed
-
- Smith MT. Mechanisms of troglitazone hepatotoxicity. Chem Res Toxicol. 2003;16:679–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

