Isoflurane reversibly destabilizes hippocampal dendritic spines by an actin-dependent mechanism
- PMID: 25068870
- PMCID: PMC4113311
- DOI: 10.1371/journal.pone.0102978
Isoflurane reversibly destabilizes hippocampal dendritic spines by an actin-dependent mechanism
Abstract
General anesthetics produce a reversible coma-like state through modulation of excitatory and inhibitory synaptic transmission. Recent evidence suggests that anesthetic exposure can also lead to sustained cognitive dysfunction. However, the subcellular effects of anesthetics on the structure of established synapses are not known. We investigated effects of the widely used volatile anesthetic isoflurane on the structural stability of hippocampal dendritic spines, a postsynaptic structure critical to excitatory synaptic transmission in learning and memory. Exposure to clinical concentrations of isoflurane induced rapid and non-uniform shrinkage and loss of dendritic spines in mature cultured rat hippocampal neurons. Spine shrinkage was associated with a reduction in spine F-actin concentration. Spine loss was prevented by either jasplakinolide or cytochalasin D, drugs that prevent F-actin disassembly. Isoflurane-induced spine shrinkage and loss were reversible upon isoflurane elimination. Thus, isoflurane destabilizes spine F-actin, resulting in changes to dendritic spine morphology and number. These findings support an actin-based mechanism for isoflurane-induced alterations of synaptic structure in the hippocampus. These reversible alterations in dendritic spine structure have important implications for acute anesthetic effects on excitatory synaptic transmission and synaptic stability in the hippocampus, a locus for anesthetic-induced amnesia, and have important implications for anesthetic effects on synaptic plasticity.
Conflict of interest statement
Figures
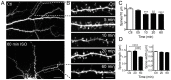



References
-
- Hemmings HC Jr, Yan W, Westphalen RI, Ryan TA (2005) The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol 67: 1591–1599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

