Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells
- PMID: 25069624
- PMCID: PMC4194154
- DOI: 10.1111/jth.12677
Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells
Abstract
Background: Extracellular histones exert part of their prothrombotic activity through the stimulation of blood cells. Besides platelets, histones can bind to red blood cells (RBCs), which are important contributors to thrombogenesis, but little is known about the functional consequences of this interaction.
Objectives: To evaluate the effect of histones on the procoagulant potential of human RBCs with particular regard to the expression of surface phosphatidylserine (PS).
Methods: PS exposure on human RBCs treated with a natural mixture of histones or recombinant individual histones was evaluated with fluorescein isothiocyanate-annexin-V binding and measured with flow cytometry. Calcium influx in RBCs loaded with the calcium-sensitive fluorophore Fluo-4 AM was assessed with flow cytometry. The procoagulant potential of histone-treated RBCs was evaluated with a purified prothrombinase assay and a one-stage plasma recalcification clotting test.
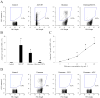
Results: Natural histones induced PS exposure on RBCs in a dose-dependent manner, and neutralization or cleavage of histones by heparin or activated protein C, respectively, abolished PS externalization. H4 was mainly responsible for the stimulating activity of histones, whereas the other subtypes were almost ineffective. Similarly, natural histones and H4 induced influx of calcium into RBCs, whereas the other individual histones did not. Histone-induced exposure of PS on RBCs translated into increased prothrombinase complex-mediated prothrombin activation and accelerated fibrin formation in plasma.
Conclusions: Histones induce RBCs to express a procoagulant phenotype through the externalization of PS. This finding provides new insights into the prothrombotic activity of extracellular histones.
Keywords: blood coagulation; erythrocytes; histones; inflammation; phosphatidylserine.
© 2014 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
C. T. Esmon holds a patent on extracellular histones as biomarkers for prognosis and molecular targets for therapy (US Patent No. 8,716,218 B2).
All other authors state that they have no conflict of interest.
Figures

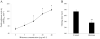
References
-
- Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

