Pharmacokinetic interaction between pyronaridine-artesunate and metoprolol
- PMID: 25070091
- PMCID: PMC4187956
- DOI: 10.1128/AAC.02716-14
Pharmacokinetic interaction between pyronaridine-artesunate and metoprolol
Abstract
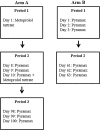
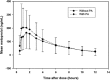
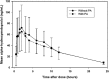
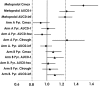
The objectives of this study were to characterize any drug-drug interaction between the antimalarial Pyramax (pyronaridine-artesunate [PA]) and the CYP2D6 probe substrate metoprolol and to assess the safety of 60-day or 90-day PA redosing, particularly with regard to liver biochemistry parameters. Healthy adult subjects were randomized to arm A (n = 26) or arm B (n = 30), with the arm A subjects administered 100 mg metoprolol tartrate in the first period, 100 mg metoprolol tartrate with the third of three daily doses of PA in the second period, and three daily doses of PA alone in the 90-day redosing period. The arm B subjects received the three-day PA regimen in the first period, with redosing of the regimen after 60 days in the second period. The noncompartmental pharmacokinetic parameters were computed for metoprolol, its metabolite alpha-hydroxymetoprolol, and pyronaridine. The coadministration of metoprolol and PA was associated with an average 47.93% (90% confidence interval [CI], 30.52, 67.66) increase in the maximum concentration of metoprolol and a 25.60% (90% CI, 15.78, 36.25) increase in the metoprolol area under the concentration-time curve from time zero to the last quantifiable concentration obtained (AUC0-t); these increases most likely resulted from pyronaridine-mediated CYP2D6 inhibition. No interaction effect of metoprolol with pyronaridine was apparent. Following dosing with PA, some subjects experienced rises in liver function tests above the upper limit of normal during the first few days following PA administration. All such elevations resolved typically within 10 days, and up to 30 days at most. In subjects who were redosed, the incidences of alanine aminotransferase (ALT) or aspartate transaminase (AST) level elevations were similar on the first and second administrations, with no marked difference between the 60-day and 90-day redosing.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Duparc S, Borghini-Fuhrer I, Craft CJ, Arbe-Barnes S, Miller RM, Shin CS, Fleckenstein L. 2013. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar. J. 12:70. 10.1186/1475-2875-12-70 - DOI - PMC - PubMed
-
- Naik H, Imming P, Schmidt MS, Murry DJ, Fleckenstein L. 2007. Development and validation of a liquid chromatography-mass spectrometry assay for the determination of pyronaridine in human blood for application to clinical pharmacokinetic studies. J. Pharm. Biomed. Anal. 45:112–119. 10.1016/j.jpba.2007.06.018 - DOI - PubMed
-
- Naik H, Murry DJ, Kirsch LE, Fleckenstein L. 2005. Development and validation of a high-performance liquid chromatography-mass spectroscopy assay for determination of artesunate and dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 816:233–242. 10.1016/j.jchromb.2004.11.042 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

