Sigma S-dependent antioxidant defense protects stationary-phase Escherichia coli against the bactericidal antibiotic gentamicin
- PMID: 25070093
- PMCID: PMC4187989
- DOI: 10.1128/AAC.03683-14
Sigma S-dependent antioxidant defense protects stationary-phase Escherichia coli against the bactericidal antibiotic gentamicin
Abstract
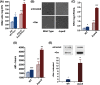
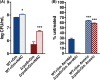
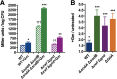
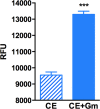
Stationary-phase bacteria are important in disease. The σ(s)-regulated general stress response helps them become resistant to disinfectants, but the role of σ(s) in bacterial antibiotic resistance has not been elucidated. Loss of σ(s) rendered stationary-phase Escherichia coli more sensitive to the bactericidal antibiotic gentamicin (Gm), and proteomic analysis suggested involvement of a weakened antioxidant defense. Use of the psfiA genetic reporter, 3'-(p-hydroxyphenyl) fluorescein (HPF) dye, and Amplex Red showed that Gm generated more reactive oxygen species (ROS) in the mutant. HPF measurements can be distorted by cell elongation, but Gm did not affect stationary-phase cell dimensions. Coadministration of the antioxidant N-acetyl cysteine (NAC) decreased drug lethality particularly in the mutant, as did Gm treatment under anaerobic conditions that prevent ROS formation. Greater oxidative stress, due to insufficient quenching of endogenous ROS and/or respiration-linked electron leakage, therefore contributed to the greater sensitivity of the mutant; infection by a uropathogenic strain in mice showed this to be the case also in vivo. Disruption of antioxidant defense by eliminating the quencher proteins, SodA/SodB and KatE/SodA, or the pentose phosphate pathway proteins, Zwf/Gnd and TalA, which provide NADPH for ROS decomposition, also generated greater oxidative stress and killing by Gm. Thus, besides its established mode of action, Gm also kills stationary-phase bacteria by generating oxidative stress, and targeting the antioxidant defense of E. coli can enhance its efficacy. Relevant aspects of the current controversy on the role of ROS in killing by bactericidal drugs of exponential-phase bacteria, which represent a different physiological state, are discussed.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

