Population pharmacokinetics of abacavir in pregnant women
- PMID: 25070097
- PMCID: PMC4187964
- DOI: 10.1128/AAC.03469-14
Population pharmacokinetics of abacavir in pregnant women
Abstract
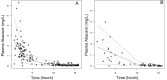
For the first time, a population approach was used to describe abacavir (ABC) pharmacokinetics in HIV-infected pregnant and nonpregnant women. A total of 266 samples from 150 women were obtained. No covariate effect (from age, body weight, pregnancy, or gestational age) on ABC pharmacokinetics was found. Thus, it seems unnecessary to adapt the ABC dosing regimen during pregnancy.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- World Health Organization. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland - PubMed
-
- Zhao W, Cella M, Della Pasqua O, Burger D, Jacqz-Aigrain E, Pediatric European Network for Treatment of AIDS (PENTA) 15 Study Group 2012. Population pharmacokinetics and maximum a posteriori probability Bayesian estimator of abacavir: application of individualized therapy in HIV-infected infants and toddlers. Br. J. Clin. Pharmacol. 73:641–650. 10.1111/j.1365-2125.2011.04121.x - DOI - PMC - PubMed
-
- Moyle GJ, DeJesus E, Cahn P, Castillo SA, Zhao H, Gordon DN, Craig C, Scott TR, Ziagen Once-Daily in Antiretroviral Combination Therapy (CNA30021) Study Team 2005. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J. Acquir. Immune Defic. Syndr. 38:417–425. 10.1097/01.qai.0000147521.34369.c9 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

