Interaction of Interferon gamma-induced reactive oxygen species with ceftazidime leads to synergistic killing of intracellular Burkholderia pseudomallei
- PMID: 25070108
- PMCID: PMC4187985
- DOI: 10.1128/AAC.02781-14
Interaction of Interferon gamma-induced reactive oxygen species with ceftazidime leads to synergistic killing of intracellular Burkholderia pseudomallei
Abstract
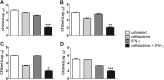
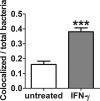
Burkholderia pseudomallei, a facultative intracellular pathogen, causes severe infections and is inherently refractory to many antibiotics. Previous studies from our group have shown that interferon gamma (IFN-γ) interacts synergistically with the antibiotic ceftazidime to kill bacteria in infected macrophages. The present study aimed to identify the underlying mechanism of that interaction. We first showed that blocking reactive oxygen species (ROS) pathways reversed IFN-γ- and ceftazidime-mediated killing, which led to our hypothesis that IFN-γ-induced ROS interacted with ceftazidime to synergistically kill Burkholderia bacteria. Consistent with this hypothesis, we also observed that buthionine sulfoximine (BSO), another inducer of ROS, could substitute for IFN-γ to similarly potentiate the effect of ceftazidime on intracellular killing. Next, we observed that IFN-γ induced ROS-mediated killing of intracellular but not extracellular bacteria. On the other hand, ceftazidime effectively reduced extracellular bacteria but was not capable of intracellular killing when applied at 10 μg/ml. We investigated the exact role of IFN-γ-induced ROS responses on intracellular bacteria and notably observed a lack of actin polymerization associated with Burkholderia bacteria in IFN-γ-treated macrophages, which led to our finding that IFN-γ-induced ROS blocks vacuolar escape. Based on these results, we propose a model in which synergistically reduced bacterial burden is achieved primarily through separate and compartmentalized killing: intracellular killing by IFN-γ-induced ROS responses and extracellular killing by ceftazidime. Our findings suggest a means of enhancing antibiotic activity against Burkholderia bacteria through combination with drugs that induce ROS pathways or otherwise target intracellular spread and/or replication of bacteria.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Dietary antioxidant seleno-L-methionine protects macrophages infected with Burkholderia thailandensis.PLoS One. 2020 Sep 3;15(9):e0238174. doi: 10.1371/journal.pone.0238174. eCollection 2020. PLoS One. 2020. PMID: 32881891 Free PMC article.
-
Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection.Antimicrob Agents Chemother. 2010 May;54(5):1785-92. doi: 10.1128/AAC.01513-09. Epub 2010 Feb 22. Antimicrob Agents Chemother. 2010. PMID: 20176901 Free PMC article.
-
Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei.Infect Immun. 1997 Oct;65(10):4108-13. doi: 10.1128/iai.65.10.4108-4113.1997. Infect Immun. 1997. PMID: 9317015 Free PMC article.
-
Induction of iNOS expression and antimicrobial activity by interferon (IFN)-beta is distinct from IFN-gamma in Burkholderia pseudomallei-infected mouse macrophages.Clin Exp Immunol. 2004 May;136(2):277-83. doi: 10.1111/j.1365-2249.2004.02445.x. Clin Exp Immunol. 2004. PMID: 15086391 Free PMC article.
-
Delineating the importance of serum opsonins and the bacterial capsule in affecting the uptake and killing of Burkholderia pseudomallei by murine neutrophils and macrophages.PLoS Negl Trop Dis. 2014 Aug 21;8(8):e2988. doi: 10.1371/journal.pntd.0002988. eCollection 2014 Aug. PLoS Negl Trop Dis. 2014. PMID: 25144195 Free PMC article.
Cited by
-
Adoptive transfer of placental ischemia-stimulated natural killer cells causes a preeclampsia-like phenotype in pregnant rats.Am J Reprod Immunol. 2021 Jun;85(6):e13386. doi: 10.1111/aji.13386. Epub 2020 Dec 27. Am J Reprod Immunol. 2021. PMID: 33315281 Free PMC article.
-
Cell free mitochondrial DNA in serum and milk associated with bovine mastitis: a pilot study.Vet Res Commun. 2018 Dec;42(4):275-282. doi: 10.1007/s11259-018-9735-z. Epub 2018 Aug 25. Vet Res Commun. 2018. PMID: 30145726
-
IL-18 binding protein (IL-18BP) as a novel radiation countermeasure after radiation exposure in mice.Sci Rep. 2020 Oct 29;10(1):18674. doi: 10.1038/s41598-020-75675-5. Sci Rep. 2020. PMID: 33122671 Free PMC article.
-
Burkholderia pseudomallei infection presenting with a lung abscess and osteomyelitis in an adult man: A case report.Medicine (Baltimore). 2018 Aug;97(35):e12145. doi: 10.1097/MD.0000000000012145. Medicine (Baltimore). 2018. PMID: 30170455 Free PMC article.
-
Dietary antioxidant seleno-L-methionine protects macrophages infected with Burkholderia thailandensis.PLoS One. 2020 Sep 3;15(9):e0238174. doi: 10.1371/journal.pone.0238174. eCollection 2020. PLoS One. 2020. PMID: 32881891 Free PMC article.
References
-
- Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377–5384. 10.1128/IAI.68.9.5377-5384.2000 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

