LKB1 and AMPK differentially regulate pancreatic β-cell identity
- PMID: 25070369
- PMCID: PMC4377859
- DOI: 10.1096/fj.14-257667
LKB1 and AMPK differentially regulate pancreatic β-cell identity
Abstract
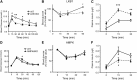
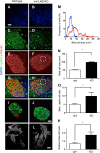
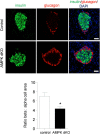
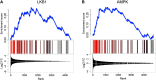
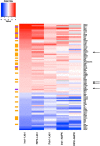
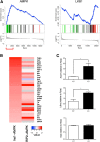
Fully differentiated pancreatic β cells are essential for normal glucose homeostasis in mammals. Dedifferentiation of these cells has been suggested to occur in type 2 diabetes, impairing insulin production. Since chronic fuel excess ("glucotoxicity") is implicated in this process, we sought here to identify the potential roles in β-cell identity of the tumor suppressor liver kinase B1 (LKB1/STK11) and the downstream fuel-sensitive kinase, AMP-activated protein kinase (AMPK). Highly β-cell-restricted deletion of each kinase in mice, using an Ins1-controlled Cre, was therefore followed by physiological, morphometric, and massive parallel sequencing analysis. Loss of LKB1 strikingly (2.0-12-fold, E<0.01) increased the expression of subsets of hepatic (Alb, Iyd, Elovl2) and neuronal (Nptx2, Dlgap2, Cartpt, Pdyn) genes, enhancing glutamate signaling. These changes were partially recapitulated by the loss of AMPK, which also up-regulated β-cell "disallowed" genes (Slc16a1, Ldha, Mgst1, Pdgfra) 1.8- to 3.4-fold (E < 0.01). Correspondingly, targeted promoters were enriched for neuronal (Zfp206; P = 1.3 × 10(-33)) and hypoxia-regulated (HIF1; P = 2.5 × 10(-16)) transcription factors. In summary, LKB1 and AMPK, through only partly overlapping mechanisms, maintain β-cell identity by suppressing alternate pathways leading to neuronal, hepatic, and other characteristics. Selective targeting of these enzymes may provide a new approach to maintaining β-cell function in some forms of diabetes.
Keywords: RNASeq; diabetes; insulin secretion; islet.
© FASEB.
Figures

References
-
- Scully T. (2012) Diabetes in numbers. Nature 485, S2–S3 - PubMed
-
- Rutter G. A., Parton L. E. (2008) The beta-cell in type 2 diabetes and in obesity. Front. Horm. Res. 36, 118–134 - PubMed
-
- Rutter G. A. (2001) Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Mol. Aspects Med. 22, 247–284 - PubMed
-
- Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 - PubMed
-
- Rahier J., Guiot Y., Goebbels R. M., Sempoux C., Henquin J. C. (2008) Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10, 32–42 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

