Light chain multiple myeloma, clinic features, responses to therapy and survival in a long-term study
- PMID: 25070574
- PMCID: PMC4237965
- DOI: 10.1186/1477-7819-12-234
Light chain multiple myeloma, clinic features, responses to therapy and survival in a long-term study
Abstract
Background: We intended to investigate the long-term clinical characteristics, responses to therapy and survival in patients with lightchain multiple myeloma (MM).
Methods: Ninety-six patients were enrolled into the study. There were 42 κ-chain MM patients and 54 λ-chain MM patients. All the patients werestage III in the Durie-Salmonstaging system. Among them, 66 patients received Velcade (bortezomib) treatment and the other 30 did not.
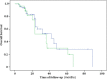
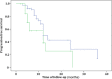
Results: The main symptoms of these patients included bone pain (77.1%), weakness and fatigue (12.5%), foamy urine (8.3%) and extramedullaryplasmocytomas (33.3%). The overall response rate (ORR) was 95.5% in patients treated with Velcade and 60%in the patients without. The median survival times were 23 months in patients treated with Velcade and 12 months in patients without. The median time of progression-free survival (PFS) was nine months in patients treated with Velcade and five months in patients without. The one-year PFS and two-year PFS were 37% and 25%, 27% and 9% for patients treated with Velcade, or without, respectively. The three-year overall survival (OS) and five-year OS were 33% and 24%, 28% and 9% for patients treated with Velcade, or without, respectively. There was no significance in OS between the two groups (P = 0.335). But there was significant difference in PFS between the two groups (P = 0.036).
Conclusions: Our long-term study demonstrated that patients with lightchain myeloma appeared to have more aggressive disease courses and poor outcomes, which could be improved by treatment with Velcade.
Figures
References
-
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

