PI3K/mTOR inhibition markedly potentiates HDAC inhibitor activity in NHL cells through BIM- and MCL-1-dependent mechanisms in vitro and in vivo
- PMID: 25070836
- PMCID: PMC4166554
- DOI: 10.1158/1078-0432.CCR-14-0034
PI3K/mTOR inhibition markedly potentiates HDAC inhibitor activity in NHL cells through BIM- and MCL-1-dependent mechanisms in vitro and in vivo
Abstract
Purpose: The aim of this study is to explore the efficacy and define mechanisms of action of coadministration of the PI3K/mTOR inhibitor BEZ235 and pan-HDAC inhibitor panobinostat in diffuse large B-cell lymphoma (DLBCL) cells.
Experimental design: Various DLBCL cells were exposed to panobinostat and BEZ235 alone or together after which apoptosis and signaling/survival pathway perturbations were monitored by flow cytometry and Western blot analysis. Genetic strategies defined the functional significance of such changes, and xenograft mouse models were used to assess tumor growth and animal survival.
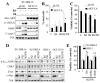
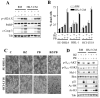
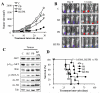
Results: Panobinostat and BEZ235 interacted synergistically in ABC-, GC-, and double-hit DLBCL cells and MCL cells but not in normal CD34(+) cells. Synergism was associated with pronounced AKT dephosphorylation, GSK3 dephosphorylation/activation, Mcl-1 downregulation, Bim upregulation, increased Bcl-2/Bcl-xL binding, diminished Bax/Bak binding to Bcl-2/Bcl-xL/Mcl-1, increased γH2A.X phosphorylation and histone H3/H4 acetylation, and abrogation of p21(CIP1) induction. BEZ235/panobinostat lethality was not susceptible to stromal/microenvironmental forms of resistance. Genetic strategies confirmed significant functional roles for AKT inactivation, Mcl-1 downregulation, Bim upregulation, and Bax/Bak in synergism. Finally, coadministration of BEZ235 with panobinostat in immunocompromised mice bearing SU-DHL4-derived tumors significantly reduced tumor growth in association with similar signaling changes observed in vitro, and combined treatment increased animal survival compared with single agents.
Conclusions: BEZ235/panobinostat exhibits potent anti-DLBCL activity, including in poor-prognosis ABC- and double-hit subtypes, but not in normal CD34(+) cells. Synergism is most likely multifactorial, involving AKT inactivation/GSK3 activation, Bim upregulation, Mcl-1 downregulation, enhanced DNA damage, and is operative in vivo. Combined PI3K/mTOR and HDAC inhibition warrants further attention in DLBCL.
©2014 American Association for Cancer Research.
Figures



Comment in
-
Team work matters: dual inhibition puts non-hodgkin lymphoma under siege.Clin Cancer Res. 2014 Dec 1;20(23):5863-5. doi: 10.1158/1078-0432.CCR-14-2055. Epub 2014 Oct 7. Clin Cancer Res. 2014. PMID: 25294900 Free PMC article.
References
-
- Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–20. - PubMed
-
- Lemoine M, Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov Med. 2010;10:462–70. - PubMed
-
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50CA142509/CA/NCI NIH HHS/United States
- P50 CA142509/CA/NCI NIH HHS/United States
- P30 CA16059/CA/NCI NIH HHS/United States
- R21 CA137823/CA/NCI NIH HHS/United States
- R01 CA100866/CA/NCI NIH HHS/United States
- P50CA130805/CA/NCI NIH HHS/United States
- R01 CA167708/CA/NCI NIH HHS/United States
- P50 CA130805/CA/NCI NIH HHS/United States
- R25 GM089614/GM/NIGMS NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- R21CA137823/CA/NCI NIH HHS/United States
- CA093738/CA/NCI NIH HHS/United States
- R01 CA093738/CA/NCI NIH HHS/United States
- CA167708/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

