Expression of TIM-3, Human β-defensin-2, and FOXP3 and Correlation with Disease Activity in Pediatric Crohn's Disease with Infliximab Therapy
- PMID: 25071071
- PMCID: PMC4413971
- DOI: 10.5009/gnl13408
Expression of TIM-3, Human β-defensin-2, and FOXP3 and Correlation with Disease Activity in Pediatric Crohn's Disease with Infliximab Therapy
Abstract
Background/aims: This study investigated the expression of T cell immunoglobulin- and mucin-domain-containing molecule 3 (TIM-3), human β-defensin (HBD)-2, forkhead box protein 3 (FOXP3), and the frequency of CD4(+) CD25(+) FOXP3(+) regulatory T cells (Tregs) in children with Crohn's disease (CD) during infliximab therapy.
Methods: We enrolled 20 CD patients who received infliximab treatment for 1 year. Peripheral blood and colonic mucosal specimens were collected from all CD patients and from healthy control individuals.
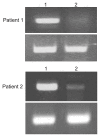
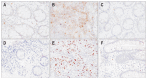
Results: A significant difference in TIM-3 mRNA expression was evident in peripheral blood mononuclear cells and colonic mucosa between CD patients before infliximab therapy and the healthy controls (p<0.001 and p=0.005, respectively). A significant difference in HBD-2 mRNA expression was found in colonic mucosa between CD patients before infliximab therapy and the healthy controls (p=0.013). In the active phase of CD, at baseline, the median percentage of T cells that were CD25(+) FOXP3(+) was 1.5% (range, 0.32% to 3.49%), which increased after inflixmab treatment for 1 year to 2.2% (range, 0.54% to 5.02%) (p=0.008).
Conclusions: Our study suggests that both the adaptive and innate immune systems are closely linked to each other in CD pathogenesis. And the results of our study indicate that it could be a useful therapeutic tool, where restoration of TIM-3, HBD-2 and the function of Tregs may repair the dysfunctional immunoregulation in CD.
Keywords: Crohn disease; Human beta-defensins-2; Infliximab; T-cell immunoglobulin- and mucin-domain-containing molecule 3; forkhead box protein 3.
Figures





Comment in
-
Cross-regulation of innate and adaptive immunity: a new perspective for the pathogenesis of inflammatory bowel disease.Gut Liver. 2015 May 23;9(3):263-4. doi: 10.5009/gnl15123. Gut Liver. 2015. PMID: 25918258 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

