Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199
- PMID: 25071121
- PMCID: PMC4162494
- DOI: 10.1200/JCO.2013.55.0491
Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199
Abstract
Purpose: Recent studies suggest that tumor-infiltrating lymphocytes (TILs) are associated with disease-free (DFS) and overall survival (OS) in operable triple-negative breast cancer (TNBC). We seek to validate the prognostic impact of TILs in primary TNBCs in two adjuvant phase III trials conducted by the Eastern Cooperative Oncology Group (ECOG).
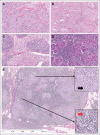
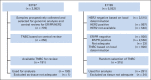
Patients and methods: Full-face hematoxylin and eosin–stained sections of 506 tumors from ECOG trials E2197 and E1199 were evaluated for density of TILs in intraepithelial (iTILs) and stromal compartments (sTILs). Patient cases of TNBC from E2197 and E1199 were randomly selected based on availability of sections. For the primary end point of DFS, association with TIL scores was determined by fitting proportional hazards models stratified on study. Secondary end points were OS and distant recurrence–free interval (DRFI). Reporting recommendations for tumor marker prognostic studies criteria were followed, and all analyses were prespecified.
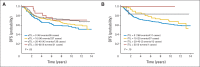
Results: The majority of 481 evaluable cancers had TILs (sTILs, 80%; iTILs, 15%). With a median follow-up of 10.6 years, higher sTIL scores were associated with better prognosis; for every 10% increase in sTILs, a 14% reduction of risk of recurrence or death (P = .02), 18% reduction of risk of distant recurrence (P = .04), and 19% reduction of risk of death (P = .01) were observed. Multivariable analysis confirmed sTILs to be an independent prognostic marker of DFS, DRFI, and OS.
Conclusion: In two national randomized clinical trials using contemporary adjuvant chemotherapy, we confirm that stromal lymphocytic infiltration constitutes a robust prognostic factor in TNBCs. Studies assessing outcomes and therapeutic efficacies should consider stratification for this parameter.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer.J Clin Oncol. 2014 Sep 20;32(27):2935-7. doi: 10.1200/JCO.2014.56.7677. J Clin Oncol. 2014. PMID: 25071115 No abstract available.
-
Tumor-infiltrating lymphocytes in breast cancer: ready for prime time?J Clin Oncol. 2015 Apr 10;33(11):1298-9. doi: 10.1200/JCO.2014.59.7286. Epub 2015 Mar 9. J Clin Oncol. 2015. PMID: 25753437 No abstract available.
-
Tumor-infiltrating lymphocytes in triple-negative breast cancer: a biomarker for use beyond prognosis?J Clin Oncol. 2015 Apr 10;33(11):1297-8. doi: 10.1200/JCO.2014.59.2808. Epub 2015 Mar 9. J Clin Oncol. 2015. PMID: 25753438 No abstract available.
References
-
- Demaria S, Pilones KA, Adams S. Cross-talk of breast cancer cells with the immune system. In: Gunduz M, Gunduz E, editors. Breast Cancer: Carcinogenesis, Cell Growth and Signalling Pathways. Rijeka, Croatia: InTech; 2011. pp. 457–482.
-
- Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–864. - PubMed
-
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. - PubMed
-
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA21076/CA/NCI NIH HHS/United States
- CA11789/CA/NCI NIH HHS/United States
- CA14958/CA/NCI NIH HHS/United States
- CA27525/CA/NCI NIH HHS/United States
- U10 CA049883/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- CA114737/CA/NCI NIH HHS/United States
- U10 CA027525/CA/NCI NIH HHS/United States
- R01CA161891/CA/NCI NIH HHS/United States
- R01 CA161891/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA014958/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- U10 CA180795/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- CA39229/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
- CA49883/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

