Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells
- PMID: 25071169
- PMCID: PMC4136565
- DOI: 10.1073/pnas.1410626111
Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells
Abstract
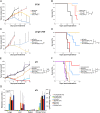
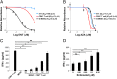
Impressive responses have been observed in patients treated with checkpoint inhibitory anti-programmed cell death-1 (PD-1) or anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) antibodies. However, immunotherapy against poorly immunogenic cancers remains a challenge. Here we report that treatment with both anti-PD-1 and anti-CTLA-4 antibodies was unable to eradicate large, modestly immunogenic CT26 tumors or metastatic 4T1 tumors. Cotreatment with epigenetic-modulating drugs and checkpoint inhibitors markedly improved treatment outcomes, curing more than 80% of the tumor-bearing mice. Functional studies revealed that the primary targets of the epigenetic modulators were myeloid-derived suppressor cells (MDSCs). A PI3K inhibitor that reduced circulating MDSCs also eradicated 4T1 tumors in 80% of the mice when combined with immune checkpoint inhibitors. Thus, cancers resistant to immune checkpoint blockade can be cured by eliminating MDSCs.
Keywords: 5-azacytidine; HDAC; entinostat; exome; methyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: Potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229(1):67–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

