Iron overload in Plasmodium berghei-infected placenta as a pathogenesis mechanism of fetal death
- PMID: 25071574
- PMCID: PMC4077027
- DOI: 10.3389/fphar.2014.00155
Iron overload in Plasmodium berghei-infected placenta as a pathogenesis mechanism of fetal death
Abstract
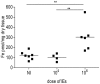
Plasmodium infection during gestation may lead to severe clinical manifestations including abortion, stillbirth, intrauterine growth retardation, and low birth weight. Mechanisms underlying such poor pregnancy outcomes are still unclear. In the animal model of severe placental malaria (PM), in utero fetal death frequently occurs and mothers often succumb to infection before or immediately after delivery. Plasmodium berghei-infected erythrocytes (IEs) continuously accumulate in the placenta, where they are then phagocytosed by fetal-derived placental cells, namely trophoblasts. Inside the phagosomes, disruption of IEs leads to the release of non-hemoglobin bound heme, which is subsequently catabolized by heme oxygenase-1 into carbon monoxide, biliverdin, and labile iron. Fine-tuned regulatory mechanisms operate to maintain iron homeostasis, preventing the deleterious effect of iron-induced oxidative stress. Our preliminary results demonstrate that iron overload in trophoblasts of P. berghei-infected placenta is associated with fetal death. Placentas which supported normally developing embryos showed no iron accumulation within the trophoblasts. Placentas from dead fetuses showed massive iron accumulation, which was associated with parasitic burden. Here we present preliminary data suggesting that disruption of iron homeostasis in trophoblasts during the course of PM is a consequence of heme accumulation after intense IE engulfment. We propose that iron overload in placenta is a pathogenic component of PM, contributing to fetal death. The mechanism through which it operates still needs to be elucidated.
Keywords: fetal death; heme; iron; pregnancy malaria; trophoblast.
Figures



Similar articles
-
Murine Model for Preclinical Studies of Var2CSA-Mediated Pathology Associated with Malaria in Pregnancy.Infect Immun. 2016 May 24;84(6):1761-1774. doi: 10.1128/IAI.01207-15. Print 2016 Jun. Infect Immun. 2016. PMID: 27045035 Free PMC article.
-
Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium-infected erythrocytes.PLoS Pathog. 2013 Jan;9(1):e1003154. doi: 10.1371/journal.ppat.1003154. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382682 Free PMC article.
-
Bradykinin Sequestration by Plasmodium berghei Infected Erythrocytes Conditions B2R Signaling and Parasite Uptake by Fetal Trophoblasts.Front Microbiol. 2018 Dec 19;9:3106. doi: 10.3389/fmicb.2018.03106. eCollection 2018. Front Microbiol. 2018. PMID: 30619185 Free PMC article.
-
Rare causes of hereditary iron overload.Semin Hematol. 2002 Oct;39(4):249-62. doi: 10.1053/shem.2002.35638. Semin Hematol. 2002. PMID: 12382200 Review.
-
Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction?Am J Obstet Gynecol. 2018 Feb;218(2S):S762-S773. doi: 10.1016/j.ajog.2017.11.567. Epub 2017 Dec 22. Am J Obstet Gynecol. 2018. PMID: 29275823 Review.
Cited by
-
Malaria and Iron Load at the First Antenatal Visit in the Rural South Kivu, Democratic Republic of the Congo: Is Iron Supplementation Safe or Could It Be Harmful?Am J Trop Med Hyg. 2018 Feb;98(2):520-523. doi: 10.4269/ajtmh.17-0585. Epub 2018 Jan 4. Am J Trop Med Hyg. 2018. PMID: 29313480 Free PMC article.
-
Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy.Front Pharmacol. 2015 Jan 13;5:291. doi: 10.3389/fphar.2014.00291. eCollection 2014. Front Pharmacol. 2015. PMID: 25628565 Free PMC article.
-
Differential roles of inflammation and apoptosis in initiation of mid-gestational abortion in malaria-infected C57BL/6 and A/J mice.Placenta. 2015 Jul;36(7):738-49. doi: 10.1016/j.placenta.2015.04.007. Epub 2015 Apr 28. Placenta. 2015. PMID: 25956987 Free PMC article.
-
A novel murine model of post-implantation malaria-induced preterm birth.PLoS One. 2022 Mar 21;17(3):e0256060. doi: 10.1371/journal.pone.0256060. eCollection 2022. PLoS One. 2022. PMID: 35312688 Free PMC article.
-
Inhibition of Heme Oxygenase-1 by Zinc Protoporphyrin IX Improves Adverse Pregnancy Outcomes in Malaria During Early Gestation.Front Immunol. 2022 May 10;13:879158. doi: 10.3389/fimmu.2022.879158. eCollection 2022. Front Immunol. 2022. PMID: 35619717 Free PMC article.
References
-
- Aisen P. (1998). Transferrin, the transferrin receptor, and the uptake of iron by cells. Met. Ions Biol. Syst. 35 585–631 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

