Vitamin D in inflammatory diseases
- PMID: 25071589
- PMCID: PMC4078458
- DOI: 10.3389/fphys.2014.00244
Vitamin D in inflammatory diseases
Abstract
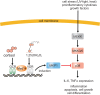
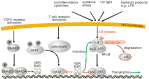
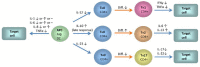
Changes in vitamin D serum levels have been associated with inflammatory diseases, such as inflammatory bowel disease (IBD), rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis (MS), atherosclerosis, or asthma. Genome- and transcriptome-wide studies indicate that vitamin D signaling modulates many inflammatory responses on several levels. This includes (i) the regulation of the expression of genes which generate pro-inflammatory mediators, such as cyclooxygenases or 5-lipoxygenase, (ii) the interference with transcription factors, such as NF-κB, which regulate the expression of inflammatory genes and (iii) the activation of signaling cascades, such as MAP kinases which mediate inflammatory responses. Vitamin D targets various tissues and cell types, a number of which belong to the immune system, such as monocytes/macrophages, dendritic cells (DCs) as well as B- and T cells, leading to individual responses of each cell type. One hallmark of these specific vitamin D effects is the cell-type specific regulation of genes involved in the regulation of inflammatory processes and the interplay between vitamin D signaling and other signaling cascades involved in inflammation. An important task in the near future will be the elucidation of the regulatory mechanisms that are involved in the regulation of inflammatory responses by vitamin D on the molecular level by the use of techniques such as chromatin immunoprecipitation (ChIP), ChIP-seq, and FAIRE-seq.
Keywords: 1α,25(OH)2D3; MKP1; NFAT; NFκB; VDR; cyclooxygenase; innate immune system; interleukins.
Figures
References
-
- Abe J., Moriya Y., Saito M., Sugawara Y., Suda T., Nishii Y. (1986). Modulation of cell growth, differentiation, and production of interleukin-3 by 1 alpha,25-dihydroxyvitamin D3 in the murine myelomonocytic leukemia cell line WEHI-3. Cancer Res. 46, 6316–6321 - PubMed
-
- Barnes P. J. (1998). Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond.) 94, 557–572 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

