Biofilm formation of mucosa-associated methanoarchaeal strains
- PMID: 25071757
- PMCID: PMC4086402
- DOI: 10.3389/fmicb.2014.00353
Biofilm formation of mucosa-associated methanoarchaeal strains
Abstract
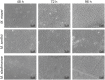
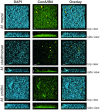
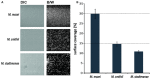
Although in nature most microorganisms are known to occur predominantly in consortia or biofilms, data on archaeal biofilm formation are in general scarce. Here, the ability of three methanoarchaeal strains, Methanobrevibacter smithii and Methanosphaera stadtmanae, which form part of the human gut microbiota, and the Methanosarcina mazei strain Gö1 to grow on different surfaces and form biofilms was investigated. All three strains adhered to the substrate mica and grew predominantly as bilayers on its surface as demonstrated by confocal laser scanning microscopy analyses, though the formation of multi-layered biofilms of Methanosphaera stadtmanae and Methanobrevibacter smithii was observed as well. Stable biofilm formation was further confirmed by scanning electron microscopy analysis. Methanosarcina mazei and Methanobrevibacter smithii also formed multi-layered biofilms in uncoated plastic μ-dishes(TM), which were very similar in morphology and reached a height of up to 40 μm. In contrast, biofilms formed by Methanosphaera stadtmanae reached only a height of 2 μm. Staining with the two lectins ConA and IB4 indicated that all three strains produced relatively low amounts of extracellular polysaccharides most likely containing glucose, mannose, and galactose. Taken together, this study provides the first evidence that methanoarchaea can develop and form biofilms on different substrates and thus, will contribute to our knowledge on the appearance and physiological role of Methanobrevibacter smithii and Methanosphaera stadtmanae in the human intestine.
Keywords: biofilms; human gut; methanoarchaea; microbiota.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

