Complement activation and regulation in preeclamptic placenta
- PMID: 25071773
- PMCID: PMC4088925
- DOI: 10.3389/fimmu.2014.00312
Complement activation and regulation in preeclamptic placenta
Abstract
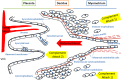
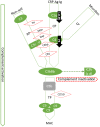
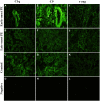
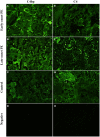
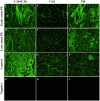
Preeclampsia (PE) is a common disorder of pregnancy originating in the placenta. We examined whether excessive activation or poor regulation of the complement system at the maternal-fetal interface could contribute to the development of PE. Location and occurrence of complement components and regulators in placentae were analyzed. Cryostat sections of placentae were processed from 7 early-onset PE (diagnosis <34 weeks of gestation), 5 late-onset PE, 10 control pregnancies, and immunostained for 6 complement activators and 6 inhibitors. Fluorescence was quantified and compared between PE and control placentae. Gene copy numbers of complement components C4A and C4B were assessed by a quantitative PCR method. Maternal C4 deficiencies (≥1 missing or non-functional C4) were most common in the early-onset PE group (71%), and more frequent in late-onset PE compared to healthy controls (60 vs. 38%). Complement C1q deposition differed significantly between control and patient groups: controls and early-onset PE patients had more C1q than late-onset PE patients (mean p = 0.01 and p = 0.005, respectively). C3 activation was analyzed by staining for C3b/iC3b and C3d. C3d was mostly specific to the basal syncytium and C3b/iC3b diffuse in other structures, but there were no clear differences between the study groups. Activated C4 and membrane-bound regulators CD55, CD46, and CD59 were observed abundantly in the syncytiotrophoblast. Syncytial knots, structures enriched in PE, stained specifically for the classical pathway inhibitor C4bp, whereas the key regulator alternative pathway, factor H (FH) showed a wider distribution in the placenta. Differences in C1q deposition between late- and early-onset PE groups may be indicative of the different etiology of PE symptoms in these patients. Irregular distribution of the complement regulators C4bp and FH in the PE placenta and a higher frequency of C4A deficiencies suggest a disturbed balance between complement activation and regulation in PE.
Keywords: complement; immunohistochemistry; innate immunity; placenta; preeclampsia; pregnancy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

