Evolutionary analysis of the cystatin family in three Schistosoma species
- PMID: 25071834
- PMCID: PMC4089355
- DOI: 10.3389/fgene.2014.00206
Evolutionary analysis of the cystatin family in three Schistosoma species
Abstract
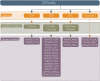
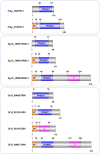
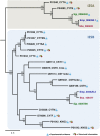
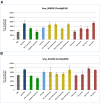
The cystatin family comprises cysteine protease inhibitors distributed in 3 subfamilies (I25A-C). Family members lacking cystatin activity are currently unclassified. Little is known about the evolution of Schistosoma cystatins, their physiological roles, and expression patterns in the parasite life cycle. The present study aimed to identify cystatin homologs in the predicted proteome of three Schistosoma species and other Platyhelminthes. We analyzed the amino acid sequence diversity focused in the identification of protein signatures and to establish evolutionary relationships among Schistosoma and experimentally validated human cystatins. Gene expression patterns were obtained from different developmental stages in Schistosoma mansoni using microarray data. In Schistosoma, only I25A and I25B proteins were identified, reflecting little functional diversification. I25C and unclassified subfamily members were not identified in platyhelminth species here analyzed. The resulting phylogeny placed cystatins in different clades, reflecting their molecular diversity. Our findings suggest that Schistosoma cystatins are very divergent from their human homologs, especially regarding the I25B subfamily. Schistosoma cystatins also differ significantly from other platyhelminth homologs. Finally, transcriptome data publicly available indicated that I25A and I25B genes are constitutively expressed thus could be essential for schistosome life cycle progression. In summary, this study provides insights into the evolution, classification, and functional diversification of cystatins in Schistosoma and other Platyhelminthes, improving our understanding of parasite biology and opening new frontiers in the identification of novel therapeutic targets against helminthiases.
Keywords: bayesian inference; function prediction; phylogenomics; proteinase inhibitor; schistosomiasis.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

