Handling tRNA introns, archaeal way and eukaryotic way
- PMID: 25071838
- PMCID: PMC4090602
- DOI: 10.3389/fgene.2014.00213
Handling tRNA introns, archaeal way and eukaryotic way
Abstract
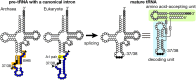
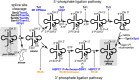
Introns are found in various tRNA genes in all the three kingdoms of life. Especially, archaeal and eukaryotic genomes are good sources of tRNA introns that are removed by proteinaceous splicing machinery. Most intron-containing tRNA genes both in archaea and eukaryotes possess an intron at a so-called canonical position, one nucleotide 3' to their anticodon, while recent bioinformatics have revealed unusual types of tRNA introns and their derivatives especially in archaeal genomes. Gain and loss of tRNA introns during various stages of evolution are obvious both in archaea and eukaryotes from analyses of comparative genomics. The splicing of tRNA molecules has been studied extensively from biochemical and cell biological points of view, and such analyses of eukaryotic systems provided interesting findings in the past years. Here, I summarize recent progresses in the analyses of tRNA introns and the splicing process, and try to clarify new and old questions to be solved in the next stages.
Keywords: archaea; eukaryote; genome; intron; splicing; tRNA.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

