Adaptive optics optical coherence tomography with dynamic retinal tracking
- PMID: 25071963
- PMCID: PMC4102363
- DOI: 10.1364/BOE.5.002262
Adaptive optics optical coherence tomography with dynamic retinal tracking
Abstract
Adaptive optics optical coherence tomography (AO-OCT) is a highly sensitive and noninvasive method for three dimensional imaging of the microscopic retina. Like all in vivo retinal imaging techniques, however, it suffers the effects of involuntary eye movements that occur even under normal fixation. In this study we investigated dynamic retinal tracking to measure and correct eye motion at KHz rates for AO-OCT imaging. A customized retina tracking module was integrated into the sample arm of the 2nd-generation Indiana AO-OCT system and images were acquired on three subjects. Analyses were developed based on temporal amplitude and spatial power spectra in conjunction with strip-wise registration to independently measure AO-OCT tracking performance. After optimization of the tracker parameters, the system was found to correct eye movements up to 100 Hz and reduce residual motion to 10 µm root mean square. Between session precision was 33 µm. Performance was limited by tracker-generated noise at high temporal frequencies.
Keywords: (110.1080) Active or adaptive optics; (120.3890) Medical optics instrumentation; (170.0110) Imaging systems; (170.4470) Ophthalmology; (170.4500) Optical coherence tomography; (330.5310) Vision - photoreceptors.
Figures
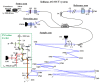


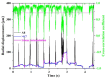







References
-
- Fernández E. J., Povazay B., Hermann B., Unterhuber A., Sattmann H., Prieto P. M., Leitgeb R., Ahnelt P., Artal P., Drexler W., “Three-dimensional adaptive optics ultrahigh-resolution optical coherence tomography using a liquid crystal spatial light modulator,” Vision Res. 45(28), 3432–3444 (2005).10.1016/j.visres.2005.08.028 - DOI - PubMed
-
- Zawadzki R. J., Jones S. M., Olivier S. S., Zhao M., Bower B. A., Izatt J. A., Choi S., Laut S., Werner J. S., “Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging,” Opt. Express 13(21), 8532–8546 (2005).10.1364/OPEX.13.008532 - DOI - PMC - PubMed
-
- Cense B., Koperda E., Brown J. M., Kocaoglu O. P., Gao W., Jonnal R. S., Miller D. T., “Volumetric retinal imaging with ultrahigh-resolution spectral-domain optical coherence tomography and adaptive optics using two broadband light sources,” Opt. Express 17(5), 4095–4111 (2009).10.1364/OE.17.004095 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
