Life without double-headed non-muscle myosin II motor proteins
- PMID: 25072053
- PMCID: PMC4083560
- DOI: 10.3389/fchem.2014.00045
Life without double-headed non-muscle myosin II motor proteins
Abstract
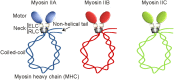
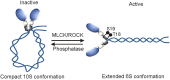
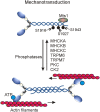
Non-muscle myosin II motor proteins (myosin IIA, myosin IIB, and myosin IIC) belong to a class of molecular motor proteins that are known to transduce cellular free-energy into biological work more efficiently than man-made combustion engines. Nature has given a single myosin II motor protein for lower eukaryotes and multiple for mammals but none for plants in order to provide impetus for their life. These specialized nanomachines drive cellular activities necessary for embryogenesis, organogenesis, and immunity. However, these multifunctional myosin II motor proteins are believed to go awry due to unknown reasons and contribute for the onset and progression of many autosomal-dominant disorders, cataract, deafness, infertility, cancer, kidney, neuronal, and inflammatory diseases. Many pathogens like HIV, Dengue, hepatitis C, and Lymphoma viruses as well as Salmonella and Mycobacteria are now known to take hostage of these dedicated myosin II motor proteins for their efficient pathogenesis. Even after four decades since their discovery, we still have a limited knowledge of how these motor proteins drive cell migration and cytokinesis. We need to enrich our current knowledge on these fundamental cellular processes and develop novel therapeutic strategies to fix mutated myosin II motor proteins in pathological conditions. This is the time to think how to relieve the hijacked myosins from pathogens in order to provide a renewed impetus for patients' life. Understanding how to steer these molecular motors in proliferating and differentiating stem cells will improve stem cell based-therapeutics development. Given the plethora of cellular activities non-muscle myosin motor proteins are involved in, their importance is apparent for human life.
Keywords: cancer; cell migration; cytokinesis; microparticles; molecular machines; motor proteins; myosin II; pathogenesis.
Figures
References
-
- Bagshaw C. R. (1993). Muscle Contraction. 2nd Edn London: Chapman and Hill
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

