Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation
- PMID: 25072388
- PMCID: PMC4114746
- DOI: 10.1371/journal.pone.0103342
Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation
Abstract
Introduction: Defining tumor from non-tumor tissue is one of the major challenges of cancer surgery. Surgeons depend on visual and tactile clues to select which tissues should be removed from a patient. Recently, we and others have hypothesized near-infrared (NIR) imaging can be used during surgery to differentiate tumors from normal tissue.
Methods: We enrolled 8 canines and 5 humans undergoing cancer surgery for NIR imaging. The patients were injected with indocyanine green (ICG), an FDA approved non-receptor specific NIR dye that accumulates in hyperpermeable tissues, 16-24 hours prior to surgery. During surgery, NIR imaging was used to discriminate the tumor from non-tumor tissue.
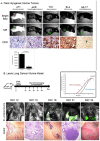
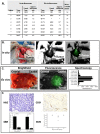
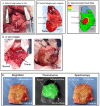
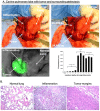
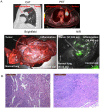
Results: NIR imaging identified all tumors with a mean signal-to-background ratio of 6.7. Optical images were useful during surgery in discriminating normal tissue from cancer. In 3 canine cases and 1 human case, the tissue surrounding the tumor was inflamed due to obstruction of the vascular supply due to mass effect. In these instances, NIR imaging could not distinguish tumor tissue from tissue that was congested, edematous and did not contain cancer.
Conclusions: This study shows that NIR imaging can identify tumors from normal tissues, provides excellent tissue contrast, and it facilitates the resection of tumors. However, in situations where there is significant peritumoral inflammation, NIR imaging with ICG is not helpful. This suggests that non-targeted NIR dyes that accumulate in hyperpermeable tissues will have significant limitations in the future, and receptor-specific NIR dyes may be necessary to overcome this problem.
Conflict of interest statement
Figures

References
-
- NCI (2009) Surveillance, Epidemiology, and End Results (SEER) Program. Nov 2009 Sub (1973-2007) <Katrian/Rita Population Adjustment> ed. Bethesda: SEER Stat Database.
-
- Klimberg VS, Harms S, Korourian S (1999) Assessing margin status. Surg Oncol 8: 77–84. - PubMed
-
- Vaidya A, Hawke C, Tiguert R, Civantos F, Soloway M (2001) Intraoperative T staging in radical retropubic prostatectomy: is it reliable? Urology 57: 949–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

