Immune regulatory properties of CD117(pos) amniotic fluid stem cells vary according to gestational age
- PMID: 25072397
- PMCID: PMC4273183
- DOI: 10.1089/scd.2014.0234
Immune regulatory properties of CD117(pos) amniotic fluid stem cells vary according to gestational age
Abstract
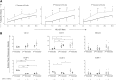
Amniotic Fluid Stem (AFS) cells are broadly multipotent fetal stem cells derived from the positive selection and ex vivo expansion of amniotic fluid CD117/c-kit(pos) cells. Considering the differentiation potential in vitro toward cell lineages belonging to the three germ layers, AFS cells have raised great interest as a new therapeutic tool, but their immune properties still need to be assessed. We analyzed the in vitro immunological properties of AFS cells from different gestational age in coculture with T, B, and natural killer (NK) cells. Nonactivated (resting) first trimester-AFS cells showed lower expression of HLA class-I molecules and NK-activating ligands than second and third trimester-AFS cells, whose features were associated with lower sensitivity to NK cell-mediated lysis. Nevertheless, inflammatory priming with interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) enhanced resistance of all AFS cell types to NK cytotoxicity. AFS cells modulated lymphocyte proliferation in a different manner according to gestational age: first trimester-AFS cells significantly inhibited T and NK cell proliferation, while second and third trimester-AFS cells were less efficient. In addition, only inflammatory-primed second trimester-AFS cells could suppress B cell proliferation, which was not affected by the first and third trimester-AFS cells. Indolamine 2,3 dioxygenase pathway was significantly involved only in T cell suppression mediated by second and third trimester-AFS cells. Overall, this study shows a number of significant quantitative differences among AFS cells of different gestational age that have to be considered in view of their clinical application.
Figures





References
-
- Prusa AR, Marton E, Rosner M, Bernaschek G. and Hengstschlager M. (2003). Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research?. Hum Reprod 18:1489–1493 - PubMed
-
- Karlmark KR, Freilinger A, Marton E, Rosner M, Lubec G. and Hengstschlager M. (2005). Activation of ectopic Oct-4 and Rex-1 promoters in human amniotic fluid cells. Int J Mol Med 16:987–992 - PubMed
-
- Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL. and Chang YJ. (2006). Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 74:545–551 - PubMed
-
- De Coppi P, Bartsch G, Jr., Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106 - PubMed
-
- Pozzobon M, Piccoli M, Schiavo AA, Atala A. and De Coppi P. (2013). Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol Biol 1035:191–198 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

