Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins
- PMID: 25073113
- PMCID: PMC7092860
- DOI: 10.1016/j.bbrc.2014.07.090
Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins
Abstract
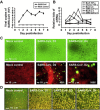
The severe acute respiratory syndrome coronavirus (SARS-CoV) still carries the potential for reemergence, therefore efforts are being made to create a vaccine as a prophylactic strategy for control and prevention. Antibody-dependent enhancement (ADE) is a mechanism through which dengue viruses, feline coronaviruses, and HIV viruses take advantage of anti-viral humoral immune responses to infect host target cells. Here we describe our observations of SARS-CoV using ADE to enhance the infectivity of a HL-CZ human promonocyte cell line. Quantitative-PCR and immunofluorescence staining results indicate that SARS-CoV is capable of replication in HL-CZ cells, and of displaying virus-induced cytopathic effects and increased levels of TNF-α, IL-4 and IL-6 two days post-infection. According to flow cytometry data, the HL-CZ cells also expressed angiotensin converting enzyme 2 (ACE2, a SARS-CoV receptor) and higher levels of the FcγRII receptor. We found that higher concentrations of anti-sera against SARS-CoV neutralized SARS-CoV infection, while highly diluted anti-sera significantly increased SARS-CoV infection and induced higher levels of apoptosis. Results from infectivity assays indicate that SARS-CoV ADE is primarily mediated by diluted antibodies against envelope spike proteins rather than nucleocapsid proteins. We also generated monoclonal antibodies against SARS-CoV spike proteins and observed that most of them promoted SARS-CoV infection. Combined, our results suggest that antibodies against SARS-CoV spike proteins may trigger ADE effects. The data raise new questions regarding a potential SARS-CoV vaccine, while shedding light on mechanisms involved in SARS pathogenesis.
Keywords: Anti-spike antibody; Antibody-dependent enhancement; Fc receptors; HL-CZ; SARS-CoV.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Chen Y.M., Liang S.Y., Shih Y.P., Chen C.Y., Lee Y.M., Chang L., Jung S.Y., Ho M.S., Liang K.Y., Chen H.Y., Chan Y.J., Chu D.C. Epidemiological and genetic correlates of severe acute respiratory syndrome coronavirus infection in the hospital with the highest nosocomial infection rate in Taiwan in 2003. J. Clin. Microbiol. 2006;44:359–365. - PMC - PubMed
-
- Barnett S.W., Burke B., Sun Y., Kan E., Legg H., Lian Y., Bost K., Zhou F., Goodsell A., Zur Megede J., Polo J., Donnelly J., Ulmer J., Otten G.R., Miller C.J., Vajdy M., Srivastava I.K. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 2010;84:5975–5985. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

