Structural basis of glycan interaction in gastroenteric viral pathogens
- PMID: 25073118
- PMCID: PMC4251800
- DOI: 10.1016/j.coviro.2014.05.008
Structural basis of glycan interaction in gastroenteric viral pathogens
Abstract
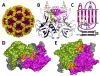
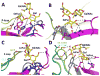
A critical event in the life cycle of a virus is its initial attachment to host cells. This involves recognition by the viruses of specific receptors on the cell surface, including glycans. Viruses typically exhibit strain-dependent variations in recognizing specific glycan receptors, a feature that contributes significantly to cell tropism, host specificity, host adaptation and interspecies transmission. Examples include influenza viruses, noroviruses, rotaviruses, and parvoviruses. Both rotaviruses and noroviruses are well known gastroenteric pathogens that are of significant global health concern. While rotaviruses, in the family Reoviridae, are the major causative agents of life-threatening diarrhea in children, noroviruses, which belong to the Caliciviridae family, cause epidemic and sporadic cases of acute gastroenteritis across all age groups. Both exhibit enormous genotypic and serotypic diversity. Consistent with this diversity each exhibits strain-dependent variations in the types of glycans they recognize for cell attachment. This chapter reviews the current status of the structural biology of such strain-dependent glycan specificities in these two families of viruses.
Copyright © 2014. Published by Elsevier B.V.
Figures


Similar articles
-
Structural basis of glycan specificity in neonate-specific bovine-human reassortant rotavirus.Nat Commun. 2015 Sep 30;6:8346. doi: 10.1038/ncomms9346. Nat Commun. 2015. PMID: 26420502 Free PMC article.
-
Investigating virus-glycan interactions using glycan microarrays.Curr Opin Virol. 2014 Aug;7:79-87. doi: 10.1016/j.coviro.2014.05.005. Epub 2014 Jul 1. Curr Opin Virol. 2014. PMID: 24995558 Free PMC article. Review.
-
Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner.J Virol. 2012 May;86(10):5584-93. doi: 10.1128/JVI.06854-11. Epub 2012 Mar 21. J Virol. 2012. PMID: 22438544 Free PMC article.
-
Fondness for sugars of enteric viruses confronts them with human glycans genetic diversity.Hum Genet. 2020 Jun;139(6-7):903-910. doi: 10.1007/s00439-019-02090-w. Epub 2019 Nov 23. Hum Genet. 2020. PMID: 31760489 Review.
-
Human Group C Rotavirus VP8*s Recognize Type A Histo-Blood Group Antigens as Ligands.J Virol. 2018 May 14;92(11):e00442-18. doi: 10.1128/JVI.00442-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29593033 Free PMC article.
Cited by
-
The effect of proteolytic enzymes and pH on GII.4 norovirus, during both interactions and non-interaction with Histo-Blood Group Antigens.Sci Rep. 2020 Oct 21;10(1):17926. doi: 10.1038/s41598-020-74728-z. Sci Rep. 2020. PMID: 33087754 Free PMC article.
-
Rotavirus Interactions With Host Intestinal Epithelial Cells.Front Immunol. 2021 Dec 22;12:793841. doi: 10.3389/fimmu.2021.793841. eCollection 2021. Front Immunol. 2021. PMID: 35003114 Free PMC article. Review.
-
Multiple Introductions and Antigenic Mismatch with Vaccines May Contribute to Increased Predominance of G12P[8] Rotaviruses in the United States.J Virol. 2018 Dec 10;93(1):e01476-18. doi: 10.1128/JVI.01476-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30333170 Free PMC article.
-
Norovirus replication, host interactions and vaccine advances.Nat Rev Microbiol. 2025 Jun;23(6):385-401. doi: 10.1038/s41579-024-01144-9. Epub 2025 Jan 17. Nat Rev Microbiol. 2025. PMID: 39824927 Free PMC article. Review.
-
Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress.Front Microbiol. 2015 Jul 1;6:659. doi: 10.3389/fmicb.2015.00659. eCollection 2015. Front Microbiol. 2015. PMID: 26191052 Free PMC article.
References
-
- Olofsson S, Bergstrom T. Glycoconjugate glycans as viral receptors. Ann Med. 2005;37:154–172. - PubMed
-
- Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. - PubMed
-
- Dormitzer PR, Sun ZY, Wagner G, Harrison SC. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 2002;21:885–897. Provided the first structural details of sialic acid binding in the VP8* of a sialidase-sensitive animal rotavirus. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

