Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice
- PMID: 25073444
- PMCID: PMC4153976
- DOI: 10.1007/s00125-014-3337-2
Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice
Abstract
Aims/hypothesis: Exosomes released from cells can transfer both functional proteins and RNAs between cells. In this study we tested the hypothesis that muscle cells might transmit specific signals during lipid-induced insulin resistance through the exosomal route.
Methods: Exosomes were collected from quadriceps muscles of C57Bl/6 mice fed for 16 weeks with either a standard chow diet (SD) or an SD enriched with 20% palm oil (HP) and from C2C12 cells exposed to 0.5 mmol/l palmitate (EXO-Post Palm), oleate (EXO-Post Oleate) or BSA (EXO-Post BSA).
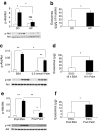
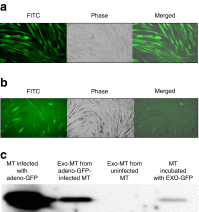
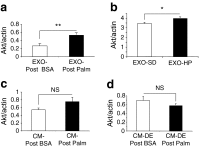
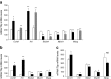
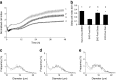
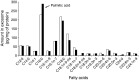
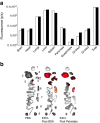
Results: HP-fed mice were obese and insulin resistant and had altered insulin-induced Akt phosphorylation in skeletal muscle (SkM). They also had reduced expression of Myod1 and Myog and increased levels of Ccnd1 mRNA, indicating that palm oil had a deep impact on SkM homeostasis in addition to insulin resistance. HP-fed mouse SkM secreted more exosomes than SD-fed mouse SkM. This was reproduced in-vitro using C2C12 cells pre-treated with palmitate, the most abundant saturated fatty acid of palm oil. Exosomes from HP-fed mice, EXO-Post Palm and EXO-Post Oleate induced myoblast proliferation and modified the expressions of genes involved in the cell cycle and muscle differentiation but did not alter insulin-induced Akt phosphorylation. Lipidomic analyses showed that exosomes from palmitate-treated cells were enriched in palmitate, indicating that exosomes likely transfer the deleterious effect of palm oil between muscle cells by transferring lipids. Muscle exosomes were incorporated into various tissues in vivo, including the pancreas and liver, suggesting that SkM could transfer specific signals through the exosomal route to key metabolic tissues.
Conclusions/interpretation: Exosomes act as 'paracrine-like' signals and modify muscle homeostasis during high-fat diets.
Figures
References
-
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev. 2012;8:457–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

