Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens
- PMID: 25073644
- PMCID: PMC4128363
- DOI: 10.1128/mBio.01534-14
Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens
Abstract
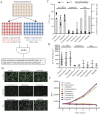
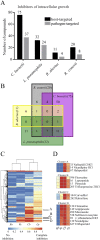
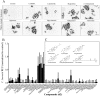
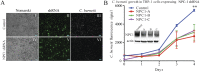
We sought a new approach to treating infections by intracellular bacteria, namely, by altering host cell functions that support their growth. We screened a library of 640 Food and Drug Administration (FDA)-approved compounds for agents that render THP-1 cells resistant to infection by four intracellular pathogens. We identified numerous drugs that are not antibiotics but were highly effective in inhibiting intracellular bacterial growth with limited toxicity to host cells. These compounds are likely to target three kinds of host functions: (i) G protein-coupled receptors, (ii) intracellular calcium signals, and (iii) membrane cholesterol distribution. The compounds that targeted G protein receptor signaling and calcium fluxes broadly inhibited Coxiella burnetii, Legionella pneumophila, Brucella abortus, and Rickettsia conorii, while those directed against cholesterol traffic strongly attenuated the intracellular growth of C. burnetii and L. pneumophila. These pathways probably support intracellular pathogen growth so that drugs that perturb them may be therapeutic candidates. Combining host- and pathogen-directed treatments is a strategy to decrease the emergence of drug-resistant intracellular bacterial pathogens. Importance: Although antibiotic treatment is often successful, it is becoming clear that alternatives to conventional pathogen-directed therapy must be developed in the face of increasing antibiotic resistance. Moreover, the costs and timing associated with the development of novel antimicrobials make repurposed FDA-approved drugs attractive host-targeted therapeutics. This paper describes a novel approach of identifying such host-targeted therapeutics against intracellular bacterial pathogens. We identified several FDA-approved drugs that inhibit the growth of intracellular bacteria, thereby implicating host intracellular pathways presumably utilized by bacteria during infection.
Copyright © 2014 Czyż et al.
Figures
Similar articles
-
Neurotransmitter System-Targeting Drugs Antagonize Growth of the Q Fever Agent, Coxiella burnetii, in Human Cells.mSphere. 2021 Aug 25;6(4):e0044221. doi: 10.1128/mSphere.00442-21. Epub 2021 Jul 7. mSphere. 2021. PMID: 34232075 Free PMC article.
-
LD-transpeptidase-mediated cell envelope remodeling enables developmental transitions and survival in Coxiella burnetii and Legionella pneumophila.J Bacteriol. 2025 Feb 20;207(2):e0024724. doi: 10.1128/jb.00247-24. Epub 2025 Jan 23. J Bacteriol. 2025. PMID: 39846729 Free PMC article.
-
Elevated Cholesterol in the Coxiella burnetii Intracellular Niche Is Bacteriolytic.mBio. 2017 Feb 28;8(1):e02313-16. doi: 10.1128/mBio.02313-16. mBio. 2017. PMID: 28246364 Free PMC article.
-
Type IVB secretion by intracellular pathogens.Traffic. 2002 Mar;3(3):178-85. doi: 10.1034/j.1600-0854.2002.030303.x. Traffic. 2002. PMID: 11886588 Review.
-
Legionella and Coxiella effectors: strength in diversity and activity.Nat Rev Microbiol. 2017 Oct;15(10):591-605. doi: 10.1038/nrmicro.2017.67. Epub 2017 Jul 17. Nat Rev Microbiol. 2017. PMID: 28713154 Review.
Cited by
-
Selective Inhibition of Coxiella burnetii Replication by the Steroid Hormone Progesterone.Infect Immun. 2020 Nov 16;88(12):e00894-19. doi: 10.1128/IAI.00894-19. Print 2020 Nov 16. Infect Immun. 2020. PMID: 32928965 Free PMC article.
-
Gefitinib Results in Robust Host-Directed Immunity Against Salmonella Infection Through Proteo-Metabolomic Reprogramming.Front Immunol. 2021 Mar 31;12:648710. doi: 10.3389/fimmu.2021.648710. eCollection 2021. Front Immunol. 2021. PMID: 33868285 Free PMC article.
-
Innovative Strategies in Drug Repurposing to Tackle Intracellular Bacterial Pathogens.Antibiotics (Basel). 2024 Sep 2;13(9):834. doi: 10.3390/antibiotics13090834. Antibiotics (Basel). 2024. PMID: 39335008 Free PMC article. Review.
-
Host-Targeted Therapeutics against Multidrug Resistant Intracellular Staphylococcus aureus.Antibiotics (Basel). 2019 Nov 28;8(4):241. doi: 10.3390/antibiotics8040241. Antibiotics (Basel). 2019. PMID: 31795127 Free PMC article. Review.
-
How Shigella Utilizes Ca(2+) Jagged Edge Signals during Invasion of Epithelial Cells.Front Cell Infect Microbiol. 2016 Feb 10;6:16. doi: 10.3389/fcimb.2016.00016. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26904514 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

