Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection
- PMID: 25073702
- PMCID: PMC4129439
- DOI: 10.7554/eLife.03206
Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection
Abstract
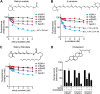
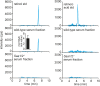
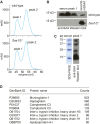
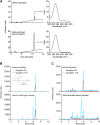
Retinol plays a vital role in the immune response to infection, yet proteins that mediate retinol transport during infection have not been identified. Serum amyloid A (SAA) proteins are strongly induced in the liver by systemic infection and in the intestine by bacterial colonization, but their exact functions remain unclear. Here we show that mouse and human SAAs are retinol binding proteins. Mouse and human SAAs bound retinol with nanomolar affinity, were associated with retinol in vivo, and limited the bacterial burden in tissues after acute infection. We determined the crystal structure of mouse SAA3 at a resolution of 2 Å, finding that it forms a tetramer with a hydrophobic binding pocket that can accommodate retinol. Our results thus identify SAAs as a family of microbe-inducible retinol binding proteins, reveal a unique protein architecture involved in retinol binding, and suggest how retinol is circulated during infection.
Keywords: acute phase response; crystal structure; retinol transport; serum amyloid A.
Copyright © 2014, Derebe et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
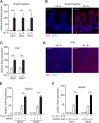
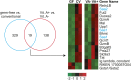
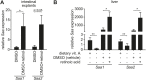

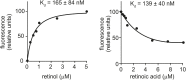



Comment in
-
An ambulance for retinol.Elife. 2014 Sep 2;3:e04246. doi: 10.7554/eLife.04246. Elife. 2014. PMID: 25182850 Free PMC article.
-
Serum amyloid A proteins take retinol for a ride.Trends Immunol. 2014 Nov;35(11):505-6. doi: 10.1016/j.it.2014.10.001. Epub 2014 Oct 18. Trends Immunol. 2014. PMID: 25443493 Free PMC article.
References
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. 2012. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D, Biological Crystallography 68:352–367. doi: 10.1107/S0907444912001308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

