Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato
- PMID: 25073705
- PMCID: PMC4149723
- DOI: 10.1104/pp.114.237388
Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato
Abstract
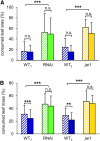
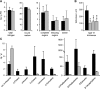
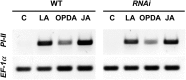
The jasmonate family of growth regulators includes the isoleucine (Ile) conjugate of jasmonic acid (JA-Ile) and its biosynthetic precursor 12-oxophytodienoic acid (OPDA) as signaling molecules. To assess the relative contribution of JA/JA-Ile and OPDA to insect resistance in tomato (Solanum lycopersicum), we silenced the expression of OPDA reductase3 (OPR3) by RNA interference (RNAi). Consistent with a block in the biosynthetic pathway downstream of OPDA, OPR3-RNAi plants contained wild-type levels of OPDA but failed to accumulate JA or JA-Ile after wounding. JA/JA-Ile deficiency in OPR3-RNAi plants resulted in reduced trichome formation and impaired monoterpene and sesquiterpene production. The loss of these JA/JA-Ile -dependent defense traits rendered them more attractive to the specialist herbivore Manduca sexta with respect to feeding and oviposition. Oviposition preference resulted from reduced levels of repellant monoterpenes and sesquiterpenes. Feeding preference, on the other hand, was caused by increased production of cis-3-hexenal acting as a feeding stimulant for M. sexta larvae in OPR3-RNAi plants. Despite impaired constitutive defenses and increased palatability of OPR3-RNAi leaves, larval development was indistinguishable on OPR3-RNAi and wild-type plants, and was much delayed compared with development on the jasmonic acid-insensitive1 (jai1) mutant. Apparently, signaling through JAI1, the tomato ortholog of the ubiquitin ligase CORONATINE INSENSITIVE1 in Arabidopsis (Arabidopsis thaliana), is required for defense, whereas the conversion of OPDA to JA/JA-Ile is not. Comparing the signaling activities of OPDA and JA/JA-Ile, we found that OPDA can substitute for JA/JA-Ile in the local induction of defense gene expression, but the production of JA/JA-Ile is required for a systemic response.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Allmann S, Baldwin IT. (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329: 1075–1078 - PubMed
-
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. (2003) Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J 34: 205–216 - PubMed
-
- Avdiushko SA, Brown GC, Dahlman DL, Hildebrand DF. (1997) Methyl jasmonate exposure induces insect resistance in cabbage and tobacco. Environ Entomol 26: 642–654
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

