Flagellar synchronization through direct hydrodynamic interactions
- PMID: 25073925
- PMCID: PMC4113993
- DOI: 10.7554/eLife.02750
Flagellar synchronization through direct hydrodynamic interactions
Abstract
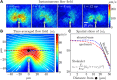
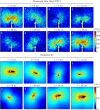
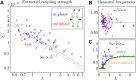
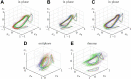
Flows generated by ensembles of flagella are crucial to development, motility and sensing, but the mechanisms behind this striking coordination remain unclear. We present novel experiments in which two micropipette-held somatic cells of Volvox carteri, with distinct intrinsic beating frequencies, are studied by high-speed imaging as a function of their separation and orientation. Analysis of time series shows that the interflagellar coupling, constrained by lack of connections between cells to be hydrodynamical, exhibits a spatial dependence consistent with theory. At close spacings it produces robust synchrony for thousands of beats, while at increasing separations synchrony is degraded by stochastic processes. Manipulation of the relative flagellar orientation reveals in-phase and antiphase states, consistent with dynamical theories. Flagellar tracking with exquisite precision reveals waveform changes that result from hydrodynamic coupling. This study proves unequivocally that flagella coupled solely through a fluid can achieve robust synchrony despite differences in their intrinsic properties.DOI: http://dx.doi.org/10.7554/eLife.02750.001.
Keywords: Volvox; flagella; synchronization.
Copyright © 2014, Brumley et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






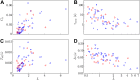
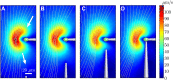
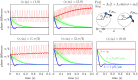
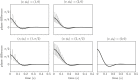
Comment in
-
Row with the flow.Elife. 2014 Jul 29;3:e03804. doi: 10.7554/eLife.03804. Elife. 2014. PMID: 25073929 Free PMC article.
References
-
- Blake JR, Chwang AT. 1973. Fundamental singularities of viscous flow. Journal of Engineering Mathematics 8:23–29. doi: 10.1007/BF02353701 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

