Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance)
- PMID: 25074878
- PMCID: PMC4161730
- DOI: 10.1200/JOP.2014.001388
Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance)
Abstract
Purpose: We evaluated associations among comorbidity, toxicity, time to relapse (TTR), and overall survival (OS) in older women with early-stage breast cancer receiving adjuvant chemotherapy.
Methods: Cancer and Leukemia Group B 49907 (Alliance) randomly assigned women ≥ 65 years old with stages I-III breast cancer to standard adjuvant chemotherapy or capecitabine. We reviewed data from 329 women who participated in the quality of life companion study CALGB 70103 and completed the Physical Health Subscale of the Older American Resources and Services Questionnaire. This questionnaire captures data on 14 comorbid conditions and the degree to which each interferes with daily activities. A comorbidity burden score was computed by multiplying the total number of conditions by each condition's level of interference with function. Outcomes were grade 3 to 5 toxicity, TTR, and OS. Logistic regression was used to evaluate associations between comorbidity and toxicity, and Cox proportional hazards models for TTR and survival.
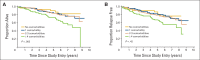
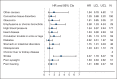
Results: Number of comorbidities ranged from 0 to 10 (median 2); the comorbidity burden score ranged from 0 to 25 (median 3). The most common conditions were arthritis (58%) and hypertension (55%). Comorbidity was associated with shorter OS, but not with toxicity or TTR. The hazard of death increased by 18% for each comorbidity (hazard ratio [HR] = 1.18, 95% CI = 1.06 to 1.33) after adjusting for age, tumor size, treatment, node and receptor status. Comorbidity burden score was similarly associated with OS (HR = 1.08; 95% CI, 1.03 to 1.14).
Conclusions: Among older women enrolled onto a clinical trial, comorbidity was associated with shorter OS, but not toxicity or relapse.
Copyright © 2014 by American Society of Clinical Oncology.
Figures
References
-
- Hurria A, Naeim A, Elkin E, et al. Adjuvant treatment recommendations in older women with breast cancer: A survey of oncologists. Crit Rev Oncol Hematol. 2007;61:255–260. - PubMed
-
- Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. - PubMed
-
- Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- K23AG038361/AG/NIA NIH HHS/United States
- CA76001/CA/NCI NIH HHS/United States
- K23 AG038361/AG/NIA NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA076001/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA025224/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

