p53Ψ is a transcriptionally inactive p53 isoform able to reprogram cells toward a metastatic-like state
- PMID: 25074920
- PMCID: PMC4136628
- DOI: 10.1073/pnas.1321640111
p53Ψ is a transcriptionally inactive p53 isoform able to reprogram cells toward a metastatic-like state
Abstract
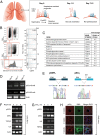
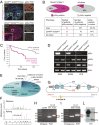
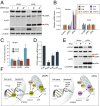
Although much is known about the underlying mechanisms of p53 activity and regulation, the factors that influence the diversity and duration of p53 responses are not well understood. Here we describe a unique mode of p53 regulation involving alternative splicing of the TP53 gene. We found that the use of an alternative 3' splice site in intron 6 generates a unique p53 isoform, dubbed p53Ψ. At the molecular level, p53Ψ is unable to bind to DNA and does not transactivate canonical p53 target genes. However, like certain p53 gain-of-function mutants, p53Ψ attenuates the expression of E-cadherin, induces expression of markers of the epithelial-mesenchymal transition, and enhances the motility and invasive capacity of cells through a unique mechanism involving the regulation of cyclophilin D activity, a component of the mitochondrial inner pore permeability. Hence, we propose that p53Ψ encodes a separation-of-function isoform that, although lacking canonical p53 tumor suppressor/transcriptional activities, is able to induce a prometastatic program in a transcriptionally independent manner.
Keywords: cancer; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Discovery of a p53 variant that controls metastasis.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11576-7. doi: 10.1073/pnas.1405580111. Epub 2014 Jul 31. Proc Natl Acad Sci U S A. 2014. PMID: 25082893 Free PMC article. No abstract available.
Similar articles
-
Tumor-suppressive p53 Signaling Empowers Metastatic Inhibitor KLF17-dependent Transcription to Overcome Tumorigenesis in Non-small Cell Lung Cancer.J Biol Chem. 2015 Aug 28;290(35):21336-51. doi: 10.1074/jbc.M114.635730. Epub 2015 Apr 24. J Biol Chem. 2015. PMID: 25911104 Free PMC article.
-
Isoform expression of CD44 adhesion molecules, Bcl-2, p53 and Ki-67 proteins in lung cancer.Tumour Biol. 2001 Jan-Feb;22(1):45-53. doi: 10.1159/000030154. Tumour Biol. 2001. PMID: 11054026
-
TP53 exon-6 truncating mutations produce separation of function isoforms with pro-tumorigenic functions.Elife. 2016 Oct 19;5:e17929. doi: 10.7554/eLife.17929. Elife. 2016. PMID: 27759562 Free PMC article.
-
p53: link to the past, bridge to the future.Genes Dev. 2005 Sep 15;19(18):2091-9. doi: 10.1101/gad.1362905. Genes Dev. 2005. PMID: 16166374 Review. No abstract available.
-
Mutant p53 on the Path to Metastasis.Trends Cancer. 2020 Jan;6(1):62-73. doi: 10.1016/j.trecan.2019.11.004. Epub 2019 Dec 16. Trends Cancer. 2020. PMID: 31952783 Free PMC article. Review.
Cited by
-
Mutant p53 exerts oncogenic functions by modulating cancer cell metabolism.Mol Cell Oncol. 2014 Dec 23;1(3):e963441. doi: 10.4161/23723548.2014.963441. eCollection 2014 Jul-Sep. Mol Cell Oncol. 2014. PMID: 27308343 Free PMC article.
-
Targeted splicing therapy: new strategies for colorectal cancer.Front Oncol. 2023 Aug 17;13:1222932. doi: 10.3389/fonc.2023.1222932. eCollection 2023. Front Oncol. 2023. PMID: 37664052 Free PMC article. Review.
-
The Δ133p53 isoform and its mouse analogue Δ122p53 promote invasion and metastasis involving pro-inflammatory molecules interleukin-6 and CCL2.Oncogene. 2016 Sep 22;35(38):4981-9. doi: 10.1038/onc.2016.45. Epub 2016 Mar 21. Oncogene. 2016. PMID: 26996665
-
Context-dependent roles for ubiquitous mitochondrial creatine kinase CKMT1 in breast cancer progression.Cell Rep. 2024 Apr 23;43(4):114121. doi: 10.1016/j.celrep.2024.114121. Epub 2024 Apr 12. Cell Rep. 2024. PMID: 38615320 Free PMC article.
-
Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production.Sci Signal. 2017 Mar 28;10(472):eaag2588. doi: 10.1126/scisignal.aag2588. Sci Signal. 2017. PMID: 28351946 Free PMC article.
References
-
- Junttila MR, Evan GI. p53—A Jack of all trades but master of none. Nat Rev Cancer. 2009;9(11):821–829. - PubMed
-
- Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. - PubMed
-
- Aldaz CM, et al. Serial analysis of gene expression in normal p53 null mammary epithelium. Oncogene. 2002;21(41):6366–6376. - PubMed
-
- Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. - PubMed
-
- Meletis K, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–369. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

