Molecular mechanisms of cellular cholesterol efflux
- PMID: 25074931
- PMCID: PMC4148835
- DOI: 10.1074/jbc.R114.583658
Molecular mechanisms of cellular cholesterol efflux
Abstract
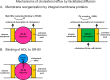
Most types of cells in the body do not express the capability of catabolizing cholesterol, so cholesterol efflux is essential for homeostasis. For instance, macrophages possess four pathways for exporting free (unesterified) cholesterol to extracellular high density lipoprotein (HDL). The passive processes include simple diffusion via the aqueous phase and facilitated diffusion mediated by scavenger receptor class B, type 1 (SR-BI). Active pathways are mediated by the ATP-binding cassette (ABC) transporters ABCA1 and ABCG1, which are membrane lipid translocases. The efflux of cellular phospholipid and free cholesterol to apolipoprotein A-I promoted by ABCA1 is essential for HDL biogenesis. Current understanding of the molecular mechanisms involved in these four efflux pathways is presented in this minireview.
Keywords: ABC Transporter; Cholesterol; Efflux; High Density Lipoprotein (HDL); Macrophage; Phospholipid; Plasma Membrane; Scavenger Receptor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Miller N. E. (1987) Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am. Heart J. 113, 589–597 - PubMed
-
- Rosenson R. S., Brewer H. B., Jr., Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., Remaley A. T., Rothblat G. H., Tall A. R., Yvan-Charvet L. (2012) Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

