Room-temperature macromolecular serial crystallography using synchrotron radiation
- PMID: 25075341
- PMCID: PMC4107920
- DOI: 10.1107/S2052252514010070
Room-temperature macromolecular serial crystallography using synchrotron radiation
Abstract
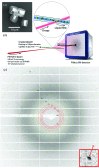
A new approach for collecting data from many hundreds of thousands of microcrystals using X-ray pulses from a free-electron laser has recently been developed. Referred to as serial crystallography, diffraction patterns are recorded at a constant rate as a suspension of protein crystals flows across the path of an X-ray beam. Events that by chance contain single-crystal diffraction patterns are retained, then indexed and merged to form a three-dimensional set of reflection intensities for structure determination. This approach relies upon several innovations: an intense X-ray beam; a fast detector system; a means to rapidly flow a suspension of crystals across the X-ray beam; and the computational infrastructure to process the large volume of data. Originally conceived for radiation-damage-free measurements with ultrafast X-ray pulses, the same methods can be employed with synchrotron radiation. As in powder diffraction, the averaging of thousands of observations per Bragg peak may improve the ratio of signal to noise of low-dose exposures. Here, it is shown that this paradigm can be implemented for room-temperature data collection using synchrotron radiation and exposure times of less than 3 ms. Using lysozyme microcrystals as a model system, over 40 000 single-crystal diffraction patterns were obtained and merged to produce a structural model that could be refined to 2.1 Å resolution. The resulting electron density is in excellent agreement with that obtained using standard X-ray data collection techniques. With further improvements the method is well suited for even shorter exposures at future and upgraded synchrotron radiation facilities that may deliver beams with 1000 times higher brightness than they currently produce.
Keywords: CrystFEL; microfocus beamline; radiation damage; room-temperature protein crystallography; serial crystallography.
Figures
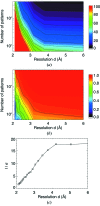
 (b) plotted as a function of resolution and of number of indexed patterns. These are both metrics of internal consistency of the data. It can be seen that consistency is improved at a given resolution with an increase in the number of indexed patterns, and consistency is improved for a given number of indexed patterns by limiting the data to lower resolution. (c) Signal-to-noise ratio [I/σ(I)] of the merged data, averaged in resolution shells, plotted as a function of resolution. I/σ(I) of each reflection is defined as the mean counts divided by the standard error of those counts (White et al., 2012 ▶).
(b) plotted as a function of resolution and of number of indexed patterns. These are both metrics of internal consistency of the data. It can be seen that consistency is improved at a given resolution with an increase in the number of indexed patterns, and consistency is improved for a given number of indexed patterns by limiting the data to lower resolution. (c) Signal-to-noise ratio [I/σ(I)] of the merged data, averaged in resolution shells, plotted as a function of resolution. I/σ(I) of each reflection is defined as the mean counts divided by the standard error of those counts (White et al., 2012 ▶).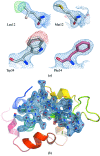
References
-
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

