Spiroindolone KAE609 for falciparum and vivax malaria
- PMID: 25075833
- PMCID: PMC4143746
- DOI: 10.1056/NEJMoa1315860
Spiroindolone KAE609 for falciparum and vivax malaria
Abstract
Background: KAE609 (cipargamin; formerly NITD609, Novartis Institute for Tropical Diseases) is a new synthetic antimalarial spiroindolone analogue with potent, dose-dependent antimalarial activity against asexual and sexual stages of Plasmodium falciparum.
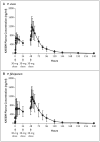
Methods: We conducted a phase 2, open-label study at three centers in Thailand to assess the antimalarial efficacy, safety, and adverse-event profile of KAE609, at a dose of 30 mg per day for 3 days, in two sequential cohorts of adults with uncomplicated P. vivax malaria (10 patients) or P. falciparum malaria (11). The primary end point was the parasite clearance time.
Results: The median parasite clearance time was 12 hours in each cohort (interquartile range, 8 to 16 hours in patients with P. vivax malaria and 10 to 16 hours in those with P. falciparum malaria). The median half-lives for parasite clearance were 0.95 hours (range, 0.68 to 2.01; interquartile range, 0.85 to 1.14) in the patients with P. vivax malaria and 0.90 hours (range, 0.68 to 1.64; interquartile range, 0.78 to 1.07) in those with P. falciparum malaria. By comparison, only 19 of 5076 patients with P. falciparum malaria (<1%) who were treated with oral artesunate in Southeast Asia had a parasite clearance half-life of less than 1 hour. Adverse events were reported in 14 patients (67%), with nausea being the most common. The adverse events were generally mild and did not lead to any discontinuations of the drug. The mean terminal half-life for the elimination of KAE609 was 20.8 hours (range, 11.3 to 37.6), supporting a once-daily oral dosing regimen.
Conclusions: KAE609, at dose of 30 mg daily for 3 days, cleared parasitemia rapidly in adults with uncomplicated P. vivax or P. falciparum malaria. (Funded by Novartis and others; ClinicalTrials.gov number, NCT01524341.).
Figures

Comment in
-
Treatment of malaria--a continuing challenge.N Engl J Med. 2014 Jul 31;371(5):474-5. doi: 10.1056/NEJMe1407026. N Engl J Med. 2014. PMID: 25075840 No abstract available.
References
-
- Malaria: fact sheet no. 94. World Health Organization; Geneva: http://www.who.int/mediacentre/factsheets/fs094/en/#
-
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
