Transplantation outcomes for severe combined immunodeficiency, 2000-2009
- PMID: 25075835
- PMCID: PMC4183064
- DOI: 10.1056/NEJMoa1401177
Transplantation outcomes for severe combined immunodeficiency, 2000-2009
Abstract
Background: The Primary Immune Deficiency Treatment Consortium was formed to analyze the results of hematopoietic-cell transplantation in children with severe combined immunodeficiency (SCID) and other primary immunodeficiencies. Factors associated with a good transplantation outcome need to be identified in order to design safer and more effective curative therapy, particularly for children with SCID diagnosed at birth.
Methods: We collected data retrospectively from 240 infants with SCID who had received transplants at 25 centers during a 10-year period (2000 through 2009).
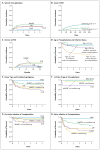
Results: Survival at 5 years, freedom from immunoglobulin substitution, and CD3+ T-cell and IgA recovery were more likely among recipients of grafts from matched sibling donors than among recipients of grafts from alternative donors. However, the survival rate was high regardless of donor type among infants who received transplants at 3.5 months of age or younger (94%) and among older infants without prior infection (90%) or with infection that had resolved (82%). Among actively infected infants without a matched sibling donor, survival was best among recipients of haploidentical T-cell-depleted transplants in the absence of any pretransplantation conditioning. Among survivors, reduced-intensity or myeloablative pretransplantation conditioning was associated with an increased likelihood of a CD3+ T-cell count of more than 1000 per cubic millimeter, freedom from immunoglobulin substitution, and IgA recovery but did not significantly affect CD4+ T-cell recovery or recovery of phytohemagglutinin-induced T-cell proliferation. The genetic subtype of SCID affected the quality of CD3+ T-cell recovery but not survival.
Conclusions: Transplants from donors other than matched siblings were associated with excellent survival among infants with SCID identified before the onset of infection. All available graft sources are expected to lead to excellent survival among asymptomatic infants. (Funded by the National Institute of Allergy and Infectious Diseases and others.).
Figures
References
-
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–9. - PubMed
-
- O’Reilly RJ, Dupont B, Pahwa S, et al. Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med. 1977;297:1311–8. - PubMed
-
- Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–8. - PubMed
-
- Cowan MJ, Wara DW, Weintrub PS, Pabst H, Ammann AJ. Haploidentical bone marrow transplantation for severe combined immunodeficiency disease using soybean agglutinin-negative, T-depleted marrow cells. J Clin Immunol. 1985;5:370–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2U01HL069254/HL/NHLBI NIH HHS/United States
- HL36444/HL/NHLBI NIH HHS/United States
- P01 CA023766/CA/NCI NIH HHS/United States
- 1U54AI082973/AI/NIAID NIH HHS/United States
- R13AI094943/AI/NIAID NIH HHS/United States
- CA23766/CA/NCI NIH HHS/United States
- HL085288/HL/NHLBI NIH HHS/United States
- R13 AI094943/AI/NIAID NIH HHS/United States
- U54 AI082973/AI/NIAID NIH HHS/United States
- U01 HL069254/HL/NHLBI NIH HHS/United States
- K23 HL085288/HL/NHLBI NIH HHS/United States
- R01 AI105776/AI/NIAID NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- P01 HL036444/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
