Design of the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial: Addressing the Knowledge Gaps
- PMID: 25076119
- PMCID: PMC4623322
- DOI: 10.3109/10903127.2014.936635
Design of the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial: Addressing the Knowledge Gaps
Abstract
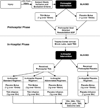
Abstract Hemorrhage and coagulopathy remain major drivers of early preventable mortality in military and civilian trauma. The development of trauma-induced coagulopathy and hyperfibrinolysis is associated with poor outcomes. Interest in the use of tranexamic acid (TXA) in hemorrhaging patients as an antifibrinolytic agent has grown recently. Additionally, several reports describe immunomodulatory effects of TXA that may confer benefit independent of its antifibrinolytic actions. A large trial demonstrated a mortality benefit for early TXA administration in patients at risk for hemorrhage; however, questions remain about the applicability in developed trauma systems and the mechanism by which TXA reduces mortality. We describe here the rationale, design, and challenges of the Study of Tranexamic Acid during Air Medical Prehospital transport (STAAMP) trial. The primary objective is to determine the effect of prehospital TXA infusion during air medical transport on 30-day mortality in patients at risk of traumatic hemorrhage. This study is a multicenter, placebo-controlled, double-blind, randomized clinical trial. The trial will enroll trauma patients with hypotension and tachycardia from 4 level I trauma center air medical transport programs. It includes a 2-phase intervention, with a prehospital and in-hospital phase to investigate multiple dosing regimens. The trial will also explore the effects of TXA on the coagulation and inflammatory response following injury. The trial will be conducted under exception for informed consent for emergency research and thus required an investigational new drug approval from the U.S. Food and Drug Administration as well as a community consultation process. It was designed to address several existing knowledge gaps and research priorities regarding TXA use in trauma.
Keywords: clinical trial; coagulopathy; hemorrhage; prehospital; tranexamic acid; trauma.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. - PubMed
-
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. - PubMed
-
- Rahbar E, Fox EE, del Junco DJ, Harvin JA, Holcomb JB, Wade CE, Schreiber MA, Rahbar MH, Bulger EM, Phelan HA, Brasel KJ, Alarcon LH, Myers JG, Cohen MJ, Muskat P, Cotton BA. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75:S16–S23. - PMC - PubMed
-
- Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, Wolf PL. The epidemiology of traumatic death: a population-based analysis. Arch Surg. 1993;128:571–575. - PubMed
-
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases