Optogenetic insights on the relationship between anxiety-related behaviors and social deficits
- PMID: 25076878
- PMCID: PMC4099964
- DOI: 10.3389/fnbeh.2014.00241
Optogenetic insights on the relationship between anxiety-related behaviors and social deficits
Abstract
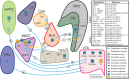
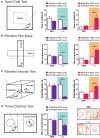
Many psychiatric illnesses are characterized by deficits in the social domain. For example, there is a high rate of co-morbidity between autism spectrum disorders and anxiety disorders. However, the common neural circuit mechanisms by which social deficits and other psychiatric disease states, such as anxiety, are co-expressed remains unclear. Here, we review optogenetic investigations of neural circuits in animal models of anxiety-related behaviors and social behaviors and discuss the important role of the amygdala in mediating aspects of these behaviors. In particular, we focus on recent evidence that projections from the basolateral amygdala (BLA) to the ventral hippocampus (vHPC) modulate anxiety-related behaviors and also alter social interaction. Understanding how this circuit influences both social behavior and anxiety may provide a mechanistic explanation for the pathogenesis of social anxiety disorder, as well as the prevalence of patients co-diagnosed with autism spectrum disorders and anxiety disorders. Furthermore, elucidating how circuits that modulate social behavior also mediate other complex emotional states will lead to a better understanding of the underlying mechanisms by which social deficits are expressed in psychiatric disease.
Keywords: anxiety; autism; basolateral amygdala; mouse models of affective disorders; optogenetics; social deficits; social interaction; ventral hippocampus.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

