Monitoring of intracranial pressure in patients with traumatic brain injury
- PMID: 25076934
- PMCID: PMC4100218
- DOI: 10.3389/fneur.2014.00121
Monitoring of intracranial pressure in patients with traumatic brain injury
Abstract
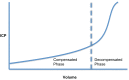
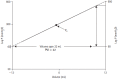
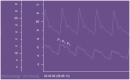
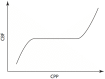
Since Monro published his observations on the nature of the contents of the intracranial space in 1783, there has been investigation of the unique relationship between the contents of the skull and the intracranial pressure (ICP). This is particularly true following traumatic brain injury (TBI), where it is clear that elevated ICP due to the underlying pathological processes is associated with a poorer clinical outcome. Consequently, there is considerable interest in monitoring and manipulating ICP in patients with TBI. The two techniques most commonly used in clinical practice to monitor ICP are via an intraventricular or intraparenchymal catheter with a microtransducer system. Both of these techniques are invasive and are thus associated with complications such as hemorrhage and infection. For this reason, significant research effort has been directed toward development of a non-invasive method to measure ICP. The principle aims of ICP monitoring in TBI are to allow early detection of secondary hemorrhage and to guide therapies that limit intracranial hypertension (ICH) and optimize cerebral perfusion. However, information from the ICP value and the ICP waveform can also be used to assess the intracranial volume-pressure relationship, estimate cerebrovascular pressure reactivity, and attempt to forecast future episodes of ICH.
Keywords: ICP; TBI; autoregulation; compliance; non-invasive monitoring.
Figures




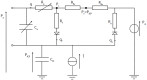



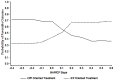
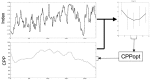
References
-
- Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg (1991) 75:S59–66
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

