Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine
- PMID: 25077142
- PMCID: PMC4097105
- DOI: 10.3389/fchem.2014.00048
Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine
Abstract
In the last 30 years we have assisted to a massive advance of nanomaterials in material science. Nanomaterials and structures, in addition to their small size, have properties that differ from those of larger bulk materials, making them ideal for a host of novel applications. The spread of nanotechnology in the last years has been due to the improvement of synthesis and characterization methods on the nanoscale, a field rich in new physical phenomena and synthetic opportunities. In fact, the development of functional nanoparticles has progressed exponentially over the past two decades. This work aims to extensively review 30 years of different strategies of surface modification and functionalization of noble metal (gold) nanoparticles, magnetic nanocrystals and semiconductor nanoparticles, such as quantum dots. The aim of this review is not only to provide in-depth insights into the different biofunctionalization and characterization methods, but also to give an overview of possibilities and limitations of the available nanoparticles.
Keywords: biofunctionalization; chemistry surface; gold nanoparticles; magnetic nanoparticles; quantum dots.
Figures



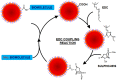
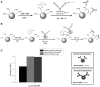
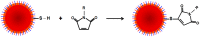
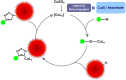

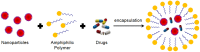
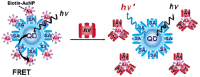
References
-
- Alivisatos A. P. (1996). Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937 10.1126/science.271.5251.933 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

