Poly(oligonucleotide)
- PMID: 25077676
- PMCID: PMC4140503
- DOI: 10.1021/ja503142s
Poly(oligonucleotide)
Abstract
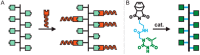
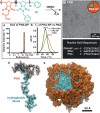
Here we report the preparation of poly(oligonucleotide) brush polymers and amphiphilic brush copolymers from nucleic acid monomers via graft-through polymerization. We describe the polymerization of PNA-norbornyl monomers to yield poly-PNA (poly(peptide nucleic acid)) via ring-opening metathesis polymerization (ROMP) with the initiator, (IMesH2)(C5H5N)2(Cl)2RuCHPh.1 In addition, we present the preparation of poly-PNA nanoparticles from amphiphilic block copolymers and describe their hybridization to a complementary single-stranded DNA (ssDNA) oligonucleotide.
Figures


References
-
- Love J. A.; Sanford M. S.; Day M. W.; Grubbs R. H. J. Am. Chem. Soc. 2003, 125, 10103. - PubMed
-
- Maynard H. D.; Okada S. Y.; Grubbs R. H. Macromolecules 2000, 33, 6239.
-
- Mammen M.; Choi S. K.; Whitesides G. M. Angew. Chem., Int. Ed. 1998, 37, 2755. - PubMed
-
- Nielsen P.; Egholm M.; Berg R.; Buchardt O. Science 1991, 254, 1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

