In vivo image analysis using iRFP transgenic mice
- PMID: 25077761
- PMCID: PMC4206735
- DOI: 10.1538/expanim.63.311
In vivo image analysis using iRFP transgenic mice
Abstract
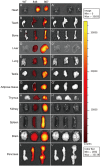
Fluorescent proteins with light wavelengths within the optical window are one of the improvements in in vivo imaging techniques. Near-infrared (NIR) fluorescent protein (iRFP) is a stable, nontoxic protein that emits fluorescence within the NIR optical window without the addition of exogenous substrate. However, studies utilizing an in vivo iRFP model have not yet been published. Here, we report the generation of transgenic iRFP mice with ubiquitous NIR fluorescence expression. iRFP expression was observed in approximately 50% of the offspring from a matings between iRFP transgenic and WT mice. The serum and blood cell indices and body weights of iRFP mice were similar to those of WT mice. Red fluorescence with an excitation wavelength of 690 nm and an emission wavelength of 713 nm was detected in both newborn and adult iRFP mice. We also detected fluorescence emission in whole organs of the iRFP mice, including the brain, heart, liver, kidney, spleen, lung, pancreas, bone, testis, thymus, and adipose tissue. Therefore, iRFP transgenic mice may therefore be a useful tool for various types of in vivo imaging.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

