Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies
- PMID: 25077917
- PMCID: PMC4167346
- DOI: 10.1007/s10928-014-9372-2
Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies
Abstract
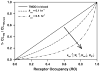
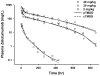
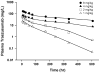
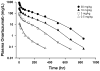
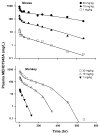
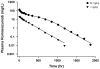
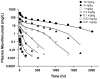
Target-mediated drug disposition (TMDD) usually accounts for nonlinear pharmacokinetics (PK) of drugs whose distribution and/or clearance are affected by their targets owing to high affinity and limited capacity. TMDD is frequently reported for monoclonal antibodies (mAb) for such reason. Minimal physiologically-based pharmacokinetic models (mPBPK), which accommodate the unique PK behaviors of mAb, provide a general approach for analyzing mAbs PK and predicting mAb interstitial concentrations in two groups of tissues. This study assessed the feasibility of incorporating TMDD into mPBPK models to consider target-binding in either plasma (cTMDD) or interstitial fluid (ISF) (pTMDD). The dose-related signature profiles of the pTMDD model reveal a parallel early decay phase, in contrast with the cTMDD model that exhibits a faster initial decline for low doses. The parallel early phase in the pTMDD model is associated with the slow perivascular extravasation of mAb, which restricts the initial decline regardless of interstitial target-mediated elimination. The cTMDD and pTMDD models both preserve the long terminal phase that is typically perceived in conventional two-compartment (2CM) and TMDD models. Having TMDD in ISF impacts the typical relationships between plasma concentrations and receptor occupancy, and between saturation of apparent nonlinear clearance and saturation of receptors. The vascular reflection coefficient (σ v ) was found to affect receptor occupancy in ISF. In the cTMDD model, saturation of nonlinear clearance is equivalent to saturation of receptors. However, in the pTMDD model, they are no longer equal and all parameters pertaining to receptors or receptor binding (R total , K D , K ss , k int ) shifts such relationships. Different TMDD models were utilized in analyzing PK for seven mAbs from digitized literature data. When the target is in plasma, the cTMDD model performed similarly to the 2CM and TMDD models, but with one less system parameter. When the target exists in ISF, the pTMDD functioned well in analyzing only plasma data to reflect interstitial target binding properties. Assigning TMDD consistent with target-expressing tissues is important to obtain reliable characterizations of receptors and receptor binding. The mPBPK model exhibits excellent feasibility in integrating TMDD not only in plasma but also in ISF.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

